当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of human V‐1 in the apo form
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-01-13 , DOI: 10.1107/s2053230x20016829 Shuichi Takeda 1 , Ryotaro Koike 2 , Takayuki Nagae 3 , Ikuko Fujiwara 4 , Akihiro Narita 1 , Yuichiro Maéda 2 , Motonori Ota 2
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-01-13 , DOI: 10.1107/s2053230x20016829 Shuichi Takeda 1 , Ryotaro Koike 2 , Takayuki Nagae 3 , Ikuko Fujiwara 4 , Akihiro Narita 1 , Yuichiro Maéda 2 , Motonori Ota 2
Affiliation
|
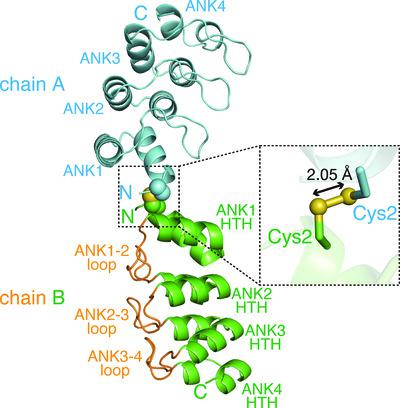
V‐1, also known as myotrophin, is a 13 kDa ankyrin‐repeat protein that binds and inhibits the heterodimeric actin capping protein (CP), which is a key regulator of cytoskeletal actin dynamics. The crystal structure of V‐1 in complex with CP revealed that V‐1 recognizes CP via residues spanning several ankyrin repeats. Here, the crystal structure of human V‐1 is reported in the absence of the specific ligand at 2.3 Å resolution. In the asymmetric unit, the crystal contains two V‐1 monomers that exhibit nearly identical structures (Cα r.m.s.d. of 0.47 Å). The overall structures of the two apo V‐1 chains are also highly similar to that of CP‐bound V‐1 (Cα r.m.s.d.s of <0.50 Å), indicating that CP does not induce a large conformational change in V‐1. Detailed structural comparisons using the computational program All Atom Motion Tree revealed that CP binding can be accomplished by minor side‐chain rearrangements of several residues. These findings are consistent with the known biological role of V‐1, in which it globally inhibits CP in the cytoplasm.
中文翻译:

apo 形式的人 V-1 的晶体结构
V-1,也称为肌营养蛋白,是一种 13 kDa 锚蛋白重复蛋白,可结合并抑制异二聚肌动蛋白加帽蛋白 (CP),后者是细胞骨架肌动蛋白动力学的关键调节因子。V-1 与 CP 复合物的晶体结构表明,V-1 通过跨越多个锚蛋白重复序列的残基识别 CP。此处,在没有特定配体的情况下以 2.3 Å 分辨率报告了人 V-1 的晶体结构。在不对称单元中,晶体包含两个具有几乎相同结构的 V-1 单体(C α rmsd 为 0.47 Å)。两条 apo V-1 链的整体结构也与 CP 结合的 V-1 高度相似(C α rmsds <0.50 Å),表明 CP 不会诱导 V-1 发生较大的构象变化。使用计算程序All Atom Motion Tree进行详细的结构比较表明,CP 结合可以通过几个残基的微小侧链重排来完成。这些发现与 V-1 已知的生物学作用一致,即它全面抑制细胞质中的 CP。
更新日期:2021-01-13
中文翻译:

apo 形式的人 V-1 的晶体结构
V-1,也称为肌营养蛋白,是一种 13 kDa 锚蛋白重复蛋白,可结合并抑制异二聚肌动蛋白加帽蛋白 (CP),后者是细胞骨架肌动蛋白动力学的关键调节因子。V-1 与 CP 复合物的晶体结构表明,V-1 通过跨越多个锚蛋白重复序列的残基识别 CP。此处,在没有特定配体的情况下以 2.3 Å 分辨率报告了人 V-1 的晶体结构。在不对称单元中,晶体包含两个具有几乎相同结构的 V-1 单体(C α rmsd 为 0.47 Å)。两条 apo V-1 链的整体结构也与 CP 结合的 V-1 高度相似(C α rmsds <0.50 Å),表明 CP 不会诱导 V-1 发生较大的构象变化。使用计算程序All Atom Motion Tree进行详细的结构比较表明,CP 结合可以通过几个残基的微小侧链重排来完成。这些发现与 V-1 已知的生物学作用一致,即它全面抑制细胞质中的 CP。



























 京公网安备 11010802027423号
京公网安备 11010802027423号