Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2021-01-13 , DOI: 10.1016/j.bbamem.2021.183566 Sarah C Bernier 1 , Marc-Antoine Millette 1 , Sarah Roy 1 , Line Cantin 1 , Ana Coutinho 2 , Christian Salesse 1
|
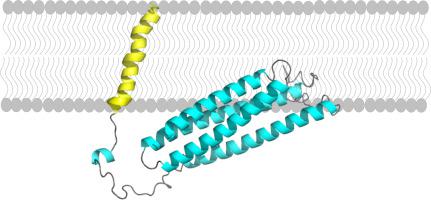
Visual phototransduction takes place in photoreceptor cells. Light absorption by rhodopsin leads to the activation of transducin as a result of the exchange of its GDP for GTP. The GTP-bound ⍺-subunit of transducin then activates phosphodiesterase (PDE), which in turn hydrolyzes cGMP leading to photoreceptor hyperpolarization. Photoreceptors return to the dark state upon inactivation of these proteins. In particular, PDE is inactivated by the protein complex R9AP/RGS9-1/Gβ5. R9AP (RGS9-1 anchor protein) is responsible for the membrane anchoring of this protein complex to photoreceptor outer segment disk membranes most likely by the combined involvement of its C-terminal hydrophobic domain as well as other types of interactions. This study thus aimed to gather information on the structure and membrane binding of the C-terminal hydrophobic segment of R9AP as well as of truncated R9AP (without its C-terminal domain, R9AP∆TM). Circular dichroism and infrared spectroscopic measurements revealed that the secondary structure of R9AP∆TM mainly includes ⍺-helical structural elements. Moreover, intrinsic fluorescence measurements of native R9AP∆TM and individual mutants lacking one tryptophan demonstrated that W79 is more buried than W173 but that they are both located in a hydrophobic environment. This method also revealed that membrane binding of R9AP∆TM does not involve regions near its tryptophan residues, while infrared spectroscopy validated its binding to lipid vesicles. Additional fluorescence measurements showed that the C-terminal segment of R9AP is membrane embedded. Maximum insertion pressure and synergy data using Langmuir monolayers suggest that interactions with specific phospholipids could be involved in the membrane binding of R9AP∆TM.
中文翻译:

结构信息和膜截断的结合- [R GS 9 -1甲nchor P rotein和其C末端疏水性链段
视觉光转导发生在感光细胞中。视紫红质的光吸收导致了转导蛋白的活化,这是由于其GDP交换为GTP的结果。然后,与GTP结合的转导蛋白的γ-亚基激活磷酸二酯酶(PDE),进而水解cGMP,导致感光器超极化。这些蛋白质失活后,感光细胞恢复到黑暗状态。特别地,PDE被蛋白质复合物R9AP / RGS9-1 /Gβ5灭活。R9AP(RGS9-1锚定蛋白)最有可能通过其C端疏水域以及其他类型的相互作用共同参与,将该蛋白复合物的膜锚定到感光器外段盘膜上。因此,本研究旨在收集有关R9AP C端疏水段以及截短的R9AP(无C端结构域R9AP∆TM)的结构和膜结合的信息。圆二色性和红外光谱测量结果表明,R9AP∆TM的二级结构主要包括β螺旋结构元素。此外,天然R9AP∆TM和缺乏一个色氨酸的单个突变体的内在荧光测量结果表明,W79比W173埋藏得更多,但它们都位于疏水环境中。该方法还表明,R9AP∆TM的膜结合不涉及色氨酸残基附近的区域,而红外光谱验证了其与脂质囊泡的结合。额外的荧光测量表明,R9AP的C末端片段被膜嵌入。



























 京公网安备 11010802027423号
京公网安备 11010802027423号