当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen Abstraction of Acetic Acid by Hydrogen Atom to Form Carboxymethyl Radical •CH2C(O)OH in Solid para-Hydrogen and Its Implication in Astrochemistry
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-01-11 , DOI: 10.1021/acsearthspacechem.0c00316 Prasad Ramesh Joshi, Kylie Chia-Yee How, Yuan-Pern Lee
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-01-11 , DOI: 10.1021/acsearthspacechem.0c00316 Prasad Ramesh Joshi, Kylie Chia-Yee How, Yuan-Pern Lee
|
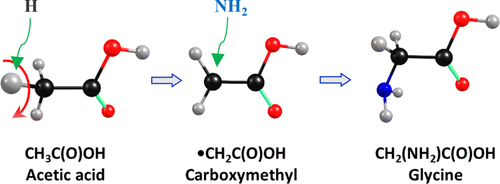
Acetic acid CH3C(O)OH attracts significant attention in interstellar chemistry because it is considered to be a potential precursor for the formation of amino acids, such as glycine and alanine. The reaction NH2 + •CH2C(O)OH within the ice mantles and on an ice surface is considered to be responsible for the formation of glycine from CH3C(O)OH. However, detailed experimental investigations are scarce. We took advantage of the unique properties of para-hydrogen (p-H2), which serves as a quantum-solid matrix host and a medium for hydrogen-atom tunneling reactions, to investigate the reaction between CH3C(O)OH and H atoms. On photolysis at 365 nm of a CH3C(O)OH/Cl2/p-H2 matrix to produce Cl atoms and subsequent infrared irradiation to produce H atoms by promoting Cl + H2 (v = 1) → H + HCl, new lines at 3581.6, 1681.0, 1455.6, 1350.7, 1180.9, 968.4, 898.4, 766.4, and 579.5 cm–1 were observed and assigned to the carboxymethyl radical, •CH2C(O)OH; the observed new spectrum agrees satisfactorily with IR intensities and scaled harmonic vibrational wavenumbers predicted with the B3LYP/aug-cc-pVTZ method. The observation of only •CH2C(O)OH as a product of H + CH3C(O)OH indicates that the reaction proceeds through H-abstraction at the CH3 moiety, whereas H-abstraction from the OH-site and other H-addition channels are unfavorable. Further H-abstraction from •CH2C(O)OH was unobserved. This previously neglected H-abstraction from CH3C(O)OH is expected to be a major channel for the formation of •CH2C(O)OH on a grain surface and bulk ice and to play an important role in the formation of glycine and other complex organic molecules (COM) in the interstellar media.
中文翻译:

氢原子萃取乙酸制氢形成固体对氢中的羧甲基自由基•CH 2 C(O)OH及其在天化学中的意义
乙酸CH 3 C(O)OH在星际化学中备受关注,因为它被认为是形成氨基酸(例如甘氨酸和丙氨酸)的潜在前体。在冰套中和在冰表面上的NH 2 +•CH 2 C(O)OH反应被认为是由CH 3 C(O)OH形成甘氨酸的原因。但是,缺乏详细的实验研究。我们利用对氢(p -H 2)的独特性质来研究CH 3之间的反应,该对氢用作量子固体基质主体和氢原子隧穿反应的介质C(O)OH和H原子。CH 3 C(O)OH / Cl 2 / p -H 2基质在365 nm处光解产生Cl原子,然后通过促进Cl + H 2(v = 1)→H + HCl进行红外辐射产生H原子,观察到了在3581.6、1681.0、1455.6、1350.7、1180.9、968.4、898.4、766.4和579.5 cm –1处的新行,并将其分配给羧甲基自由基•CH 2 C(O)OH;观测到的新光谱与IR强度和B3LYP / aug-cc-pVTZ方法预测的缩放谐波振动波数令人满意地吻合。仅观察到作为H + CH 3产物的•CH 2 C(O)OHC(O)OH表示反应是通过在CH 3部分进行H抽提进行的,而从OH-位和其他H加成通道的H抽提是不利的。未观察到从•CH 2 C(O)OH进一步吸氢。CH 3 C(O)OH的这种先前被忽略的H吸收有望成为在谷物表面和大块冰上形成•CH 2 C(O)OH的主要通道,并在形成冰的过程中发挥重要作用。星际介质中的甘氨酸和其他复杂有机分子(COM)。
更新日期:2021-01-21
中文翻译:

氢原子萃取乙酸制氢形成固体对氢中的羧甲基自由基•CH 2 C(O)OH及其在天化学中的意义
乙酸CH 3 C(O)OH在星际化学中备受关注,因为它被认为是形成氨基酸(例如甘氨酸和丙氨酸)的潜在前体。在冰套中和在冰表面上的NH 2 +•CH 2 C(O)OH反应被认为是由CH 3 C(O)OH形成甘氨酸的原因。但是,缺乏详细的实验研究。我们利用对氢(p -H 2)的独特性质来研究CH 3之间的反应,该对氢用作量子固体基质主体和氢原子隧穿反应的介质C(O)OH和H原子。CH 3 C(O)OH / Cl 2 / p -H 2基质在365 nm处光解产生Cl原子,然后通过促进Cl + H 2(v = 1)→H + HCl进行红外辐射产生H原子,观察到了在3581.6、1681.0、1455.6、1350.7、1180.9、968.4、898.4、766.4和579.5 cm –1处的新行,并将其分配给羧甲基自由基•CH 2 C(O)OH;观测到的新光谱与IR强度和B3LYP / aug-cc-pVTZ方法预测的缩放谐波振动波数令人满意地吻合。仅观察到作为H + CH 3产物的•CH 2 C(O)OHC(O)OH表示反应是通过在CH 3部分进行H抽提进行的,而从OH-位和其他H加成通道的H抽提是不利的。未观察到从•CH 2 C(O)OH进一步吸氢。CH 3 C(O)OH的这种先前被忽略的H吸收有望成为在谷物表面和大块冰上形成•CH 2 C(O)OH的主要通道,并在形成冰的过程中发挥重要作用。星际介质中的甘氨酸和其他复杂有机分子(COM)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号