当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron overload reduces adiponectin receptor expression via a ROS/FOXO1‐dependent mechanism leading to adiponectin resistance in skeletal muscle cells
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2021-01-11 , DOI: 10.1002/jcp.30240 Karam Dahyaleh 1 , Hye K Sung 1 , Michelle Prioriello 1 , Palanivel Rengasamy 1 , Nhat H Lam 1 , Jae B Kim 2 , Sean Gross 3 , Gary Sweeney 1
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2021-01-11 , DOI: 10.1002/jcp.30240 Karam Dahyaleh 1 , Hye K Sung 1 , Michelle Prioriello 1 , Palanivel Rengasamy 1 , Nhat H Lam 1 , Jae B Kim 2 , Sean Gross 3 , Gary Sweeney 1
Affiliation
|
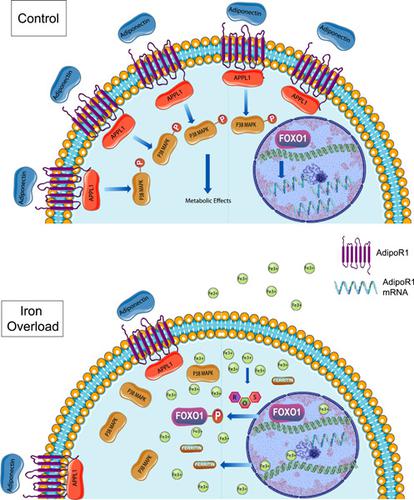
Iron overload (IO) is a common yet underappreciated finding in metabolic syndrome (MetS) patients. With the prevalence of MetS continuing to rise, it is imperative to further elucidate cellular mechanisms leading to metabolic dysfunction. Adiponectin has many beneficial effects and is a therapeutic target for the treatment of MetS and cardiovascular diseases. IO positively correlates with reduced circulating adiponectin levels yet the impact of IO on adiponectin action is unknown. Here, we established a model of IO in L6 skeletal muscle cells and found that IO‐induced adiponectin resistance. This was shown via reduced p38 mitogen‐activated protein kinase phosphorylation in response to the small molecule adiponectin receptor (AdipoR) agonist, AdipoRon, in presence of IO. This correlated with reduced messenger RNA and protein levels of AdipoR1 and its facilitative signaling binding partner, APPL1. IO caused phosphorylation, nuclear extrusion, and thus inhibition of FOXO1, a known transcription factor regulating AdipoR1 expression. The antioxidant N‐acetyl cystine attenuated the production of reactive oxygen species (ROS) by IO, and blunted its effect on FOXO1 phosphorylation and removal from the nucleus, as well as subsequent adiponectin resistance. In conclusion, our study identifies a ROS/FOXO1/AdipoR1 axis as a cause of skeletal muscle adiponectin resistance in response to IO. This new knowledge provides insight into a cellular mechanism with potential relevance to disease pathophysiology in MetS patients with IO.
中文翻译:

铁过载通过 ROS/FOXO1 依赖性机制降低脂联素受体表达,导致骨骼肌细胞中的脂联素抵抗
铁过载 (IO) 是代谢综合征 (MetS) 患者中常见但未被充分认识的发现。随着 MetS 的患病率持续上升,进一步阐明导致代谢功能障碍的细胞机制势在必行。脂联素具有许多有益作用,是治疗 MetS 和心血管疾病的治疗靶点。IO 与降低的循环脂联素水平呈正相关,但 IO 对脂联素作用的影响尚不清楚。在这里,我们在 L6 骨骼肌细胞中建立了 IO 模型,并发现 IO 诱导的脂联素抵抗。这是通过在 IO 存在下响应小分子脂联素受体 (AdipoR) 激动剂 AdipoRon 来减少 p38 丝裂原活化蛋白激酶磷酸化来显示的。这与 AdipoR1 及其促进信号结合伙伴 APPL1 的信使 RNA 和蛋白质水平降低有关。IO 引起磷酸化、核挤压,从而抑制 FOXO1,这是一种调节 AdipoR1 表达的已知转录因子。抗氧化剂 N-乙酰胱氨酸减弱了 IO 产生的活性氧 (ROS),并减弱了其对 FOXO1 磷酸化和从细胞核中去除的影响,以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。从而抑制 FOXO1,一种已知的调节 AdipoR1 表达的转录因子。抗氧化剂 N-乙酰胱氨酸减弱了 IO 产生的活性氧 (ROS),并减弱了其对 FOXO1 磷酸化和从细胞核中去除的影响,以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。从而抑制 FOXO1,一种已知的调节 AdipoR1 表达的转录因子。抗氧化剂 N-乙酰胱氨酸减弱了 IO 产生的活性氧 (ROS),并减弱了其对 FOXO1 磷酸化和从细胞核中去除的影响,以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。
更新日期:2021-01-11
中文翻译:

铁过载通过 ROS/FOXO1 依赖性机制降低脂联素受体表达,导致骨骼肌细胞中的脂联素抵抗
铁过载 (IO) 是代谢综合征 (MetS) 患者中常见但未被充分认识的发现。随着 MetS 的患病率持续上升,进一步阐明导致代谢功能障碍的细胞机制势在必行。脂联素具有许多有益作用,是治疗 MetS 和心血管疾病的治疗靶点。IO 与降低的循环脂联素水平呈正相关,但 IO 对脂联素作用的影响尚不清楚。在这里,我们在 L6 骨骼肌细胞中建立了 IO 模型,并发现 IO 诱导的脂联素抵抗。这是通过在 IO 存在下响应小分子脂联素受体 (AdipoR) 激动剂 AdipoRon 来减少 p38 丝裂原活化蛋白激酶磷酸化来显示的。这与 AdipoR1 及其促进信号结合伙伴 APPL1 的信使 RNA 和蛋白质水平降低有关。IO 引起磷酸化、核挤压,从而抑制 FOXO1,这是一种调节 AdipoR1 表达的已知转录因子。抗氧化剂 N-乙酰胱氨酸减弱了 IO 产生的活性氧 (ROS),并减弱了其对 FOXO1 磷酸化和从细胞核中去除的影响,以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。从而抑制 FOXO1,一种已知的调节 AdipoR1 表达的转录因子。抗氧化剂 N-乙酰胱氨酸减弱了 IO 产生的活性氧 (ROS),并减弱了其对 FOXO1 磷酸化和从细胞核中去除的影响,以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。从而抑制 FOXO1,一种已知的调节 AdipoR1 表达的转录因子。抗氧化剂 N-乙酰胱氨酸减弱了 IO 产生的活性氧 (ROS),并减弱了其对 FOXO1 磷酸化和从细胞核中去除的影响,以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。以及随后的脂联素抗性。总之,我们的研究将 ROS/FOXO1/AdipoR1 轴确定为响应 IO 的骨骼肌脂联素抵抗的原因。这一新知识提供了对与 IO 的 MetS 患者的疾病病理生理学潜在相关的细胞机制的洞察。


























 京公网安备 11010802027423号
京公网安备 11010802027423号