Tetrahedron Letters ( IF 1.8 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.tetlet.2020.152803 Yalin Wang , Jun Tian , Feng Zhao , Yu Chen , Bingyi Huo , Shanshan Yu , Xiaoqi Yu , Lin Pu
|
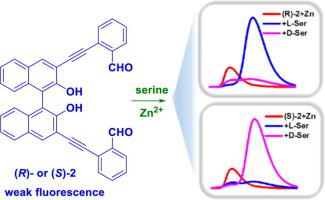
A diarylacetylene-containing 1,1′-bi-2-naphthol derivative (R)-2 was designed and synthesized. This compound in combination with Zn2+ represents the first chemoselective as well as enantioselective fluorescent probe for the biologically important amino acid serine. It was found that l-serine can greatly enhance the fluorescence of the probe at λ = 471 nm but d-serine and 17 other common amino acids cannot. An enantioselective fluorescence enhancement ratio [ef = (IL − I0)/(ID − I0) = ΔIL/ΔID] of 15 was observed for the response of (R)-2 + Zn2+ toward serine.
中文翻译:

荧光探针对丝氨酸的高度化学选择性和对映选择性识别
设计并合成了含二芳基乙炔的1,1'-联-2-萘酚衍生物(R)-2。该化合物与Zn 2+的结合代表了生物学上重要的氨基酸丝氨酸的第一个化学选择性和对映选择性荧光探针。发现1-丝氨酸可以大大增强探针在λ=471nm处的荧光,但是d-丝氨酸和其他17种常见氨基酸不能。对映体选择性的荧光增强比[EF =(I大号 - I 0)/(I d - I 0)=ΔI大号/ΔI d ]的15观察到的应答(ř)-2 + Zn 2+朝向丝氨酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号