Nano Today ( IF 17.4 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.nantod.2020.101064 Junyang Wang , Chao Zheng , Yihui Zhai , Ying Cai , Robert J. Lee , Jianming Xing , Hao Wang , Helen H. Zhu , Lesheng Teng , Yaping Li , Pengcheng Zhang
|
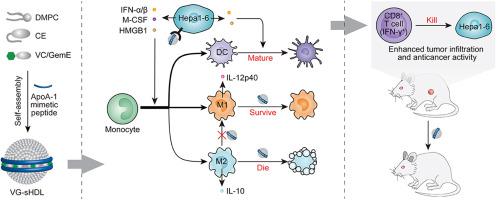
Intratumoral abundance of alternatively activated macrophages (M2) in hepatocellular carcinoma (HCC) is associated with advanced stages, poor prognosis and failure of checkpoint blockade immunotherapy. In this work, we discover that synthetic high-density lipoproteins (sHDLs) preferentially deliver cargoes into M2 and Hepa1–6 HCC cells, based on which finding we create a functional sHDL containing esterase-responsive prodrugs of vadimezan and gemcitabine (VG-sHDLs). The VG-sHDLs induce the HCC cells to release damage-associated molecular patterns (high mobility group box 1 protein and ATP) and cytokines including macrophage colony stimulating factor and type I interferons, improving monocyte differentiation into classically activated macrophages (M1) and dendritic cells. Meanwhile, the VG-sHDLs kill M2 without significant toxicity to M1. After intravenous injection, the VG-sHDLs increase intratumoral M1 as well as proinflammatory molecules such as C-X-C motif ligand 9 and 10 and interleukin 12, but reduce M2 and anti-inflammatory cytokine interleukin 10. These changes enhance the recruitment and activity of cytotoxic T lymphocytes, which eradicate HCC tumors and also establish tumor-specific immune memory that prevents recurrence. This work sheds light on the interactions between sHDL and intratumoral cells, and provides rationale for chemoimmunotherapy of HCC using conventional chemotherapeutics and emerging immune modulators.
中文翻译:

高密度脂蛋白调节肿瘤相关巨噬细胞化学疗法治疗肝细胞癌
肝细胞癌(HCC)中交替激活的巨噬细胞(M2)的瘤内丰度与晚期,预后差和检查点封锁免疫疗法失败有关。在这项工作中,我们发现合成的高密度脂蛋白(sHDLs)优先将货物递送到M2和Hepa1–6 HCC细胞,在此基础上,我们发现了一种功能性sHDL,其中包含vadimezan和吉西他滨(VG-sHDLs)的酯酶反应性前药。 。VG-sHDLs诱导HCC细胞释放与损伤相关的分子模式(高迁移率族框1蛋白和ATP)和细胞因子,包括巨噬细胞集落刺激因子和I型干扰素,改善单核细胞分化为经典激活的巨噬细胞(M1)和树突状细胞。同时,VG-sHDL杀死M2,而对M1无明显毒性。静脉注射后,VG-sHDLs增加肿瘤内M1以及促炎分子(例如CXC基序配体9和10和白介素12),但减少M2和抗炎细胞因子白介素10。这些改变增强了细胞毒性T淋巴细胞的募集和活性。可以根除HCC肿瘤,并建立防止复发的肿瘤特异性免疫记忆。这项工作阐明了sHDL与肿瘤内细胞之间的相互作用,并为使用常规化学疗法和新兴免疫调节剂进行HCC的化学免疫疗法提供了理论依据。这些改变增强了细胞毒性T淋巴细胞的募集和活性,这些细胞可根除HCC肿瘤,并建立防止复发的肿瘤特异性免疫记忆。这项工作阐明了sHDL与肿瘤内细胞之间的相互作用,并为使用常规化学疗法和新兴免疫调节剂进行HCC的化学免疫疗法提供了理论依据。这些改变增强了细胞毒性T淋巴细胞的募集和活性,这些细胞可根除HCC肿瘤,并建立防止复发的肿瘤特异性免疫记忆。这项工作阐明了sHDL与肿瘤内细胞之间的相互作用,并为使用常规化学疗法和新兴免疫调节剂进行HCC的化学免疫疗法提供了理论依据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号