Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.colsurfa.2020.126131 Yu Xin , Powei Gu , Huyan Long , Meijuan Meng , Muhammad Yaseen , Haifeng Su
|
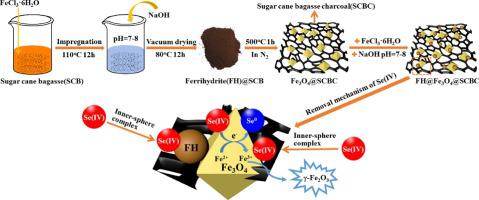
Herein we report the fabrication of ferrihydrite-loaded magnetic sugar cane bagasse charcoal adsorbent (FH@Fe3O4@SCBC) by in-situ growth method, which was in turn applied for the adsorptive removal of Se(IV) from aqueous solution. The prepared adsorbents were characterized by SEM-EDS, BET, XRD, VSM, FT-IR, and XPS analyses, and the effect of reaction temperature and pH on Se(IV) removal rate was investigated. Experimental results revealed that the maximum concentration of released Fe ions (0.20 mg L−1) was lower than the WHO highest authorized standard value for Fe in drinking water (0.30 mg L−1) indicating that FH@Fe3O4@SCBC adsorbent has good stability. Experimental results of adsorption kinetics and isotherms revealed that adsorption of Se(IV) by FH@Fe3O4@SCBC mainly occurred via chemisorption and monolayer adsorption and the maximum theoretical adsorption capacity Se(IV) was 95.15 mg g−1. The experiment on the coexisting anions showed that SO42− and PO43− exhibited a significant effect on the adsorption process of Se(IV) while Cl− and NO3− exhibited an insignificant effect. A proposed adsorption mechanism showed that Se(IV) removal included FH@Fe3O4@SCBC inner-sphere complexation, electrostatic interaction, and reduction reaction. According to VSM analysis, FH@Fe3O4@SCBC showed enhanced magnetic response thus facilitating its efficient and facile magnetic separation. Furthermore, FH@Fe3O4@SCBC realized minimal loss in adsorption activity (decreased by 11.5 %) after 10 consecutive reuses. This study provides a facile and cost-effective approach for the fabrication of highly stable FH@Fe3O4@SCBC adsorbent with high adsorption capacity, fast magnetic separation, and excellent recycling advantages making it a promising material for the remediation of Se(IV) from wastewater.
中文翻译:

负载三水铁矿的磁性甘蔗渣木炭炭吸附剂的制备,用于从水溶液中吸附去除亚硒酸盐
在这里,我们报道了通过原位生长法制备的负载三水铁矿的磁性甘蔗渣蔗糖木炭吸附剂(FH @ Fe 3 O 4 @SCBC),该吸附剂又用于从水溶液中吸附去除Se(IV)。通过SEM-EDS,BET,XRD,VSM,FT-IR和XPS分析对制备的吸附剂进行表征,研究了反应温度和pH对Se(IV)去除率的影响。实验结果表明,所释放的Fe离子的最大浓度(0.20 mg L -1)低于WHO饮用水中Fe的世界卫生组织最高授权标准值(0.30 mg L -1),表明FH @ Fe 3 O 4@SCBC吸附剂具有良好的稳定性。吸附动力学和等温线实验结果表明,FH @ Fe 3 O 4 @SCBC对Se(IV)的吸附主要通过化学吸附和单层吸附发生,最大理论吸附量Se(IV)为95.15 mg g -1。上共存阴离子实验表明,SO 4 2-和PO 4 3-表现硒的吸附过程(IV),同时CL A显著效果-和NO 3 -表现出不显着的效果。提出的吸附机理表明,Se(IV)的去除包括FH @ Fe 3 O 4。@SCBC内球络合,静电相互作用和还原反应。根据VSM分析,FH @ Fe 3 O 4 @SCBC表现出增强的磁响应,从而促进了其高效,便捷的磁分离。此外,在连续使用10次后,FH @ Fe 3 O 4 @SCBC实现了最小的吸附活性损失(降低了11.5%)。这项研究为制备高稳定性,高吸附力,快速磁分离和优异回收利用优势的高稳定性FH @ Fe 3 O 4 @SCBC吸附剂提供了一种简便且经济高效的方法,使其成为一种有望修复Se(IV)的材料。 )。


























 京公网安备 11010802027423号
京公网安备 11010802027423号