Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2021-01-11 , DOI: 10.1016/j.jcmgh.2021.01.003 Samuel M Lee 1 , Carolina M Pusec 1 , Gregory H Norris 1 , Adam De Jesus 2 , Alberto Diaz-Ruiz 3 , Jose Muratalla 1 , Andre Sarmento-Cabral 1 , Grace Guzman 4 , Brian T Layden 5 , Jose Cordoba-Chacon 1
|
Background & Aims
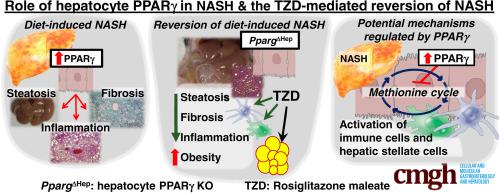
Nonalcoholic steatohepatitis (NASH) is commonly observed in patients with type 2 diabetes, and thiazolidinediones (TZD) are considered a potential therapy for NASH. Although TZD increase insulin sensitivity and partially reduce steatosis and alanine aminotransferase, the efficacy of TZD on resolving liver pathology is limited. In fact, TZD may activate peroxisome proliferator-activated receptor gamma (PPARγ) in hepatocytes and promote steatosis. Therefore, we assessed the role that hepatocyte-specific PPARγ plays in the development of NASH, and how it alters the therapeutic effects of TZD on the liver of mice with diet-induced NASH.
Methods
Hepatocyte-specific PPARγ expression was knocked out in adult mice before and after the development of NASH induced with a high fat, cholesterol, and fructose (HFCF) diet.
Results
HFCF diet increased PPARγ expression in hepatocytes, and rosiglitazone further activated PPARγ in hepatocytes of HFCF-fed mice in vivo and in vitro. Hepatocyte-specific loss of PPARγ reduced the progression of HFCF-induced NASH in male mice and increased the benefits derived from the effects of TZD on extrahepatic tissues and non-parenchymal cells. RNAseq and metabolomics indicated that HFCF diet promoted inflammation and fibrogenesis in a hepatocyte PPARγ–dependent manner and was associated with dysregulation of hepatic metabolism. Specifically, hepatocyte-specific loss of PPARγ plays a positive role in the regulation of methionine metabolism, and that could reduce the progression of NASH.
Conclusions
Because of the negative effect of hepatocyte PPARγ in NASH, inhibition of mechanisms promoted by endogenous PPARγ in hepatocytes may represent a novel strategy that increases the efficiency of therapies for NAFLD.
中文翻译:

肝细胞特异性 PPARγ 损失保护小鼠免受 NASH 的侵害并增加罗格列酮在肝脏中的治疗效果
背景与目标
非酒精性脂肪性肝炎 (NASH) 常见于 2 型糖尿病患者,噻唑烷二酮 (TZD) 被认为是 NASH 的潜在疗法。尽管 TZD 增加了胰岛素敏感性并部分降低了脂肪变性和丙氨酸氨基转移酶,但 TZD 在解决肝脏病变方面的功效有限。事实上,TZD 可能激活肝细胞中的过氧化物酶体增殖物激活受体 γ (PPARγ) 并促进脂肪变性。因此,我们评估了肝细胞特异性 PPARγ 在 NASH 发展中的作用,以及它如何改变 TZD 对饮食诱导 NASH 小鼠肝脏的治疗作用。
方法
在高脂肪、胆固醇和果糖 (HFCF) 饮食诱导的 NASH 发展前后,成年小鼠的肝细胞特异性 PPARγ 表达被敲除。
结果
HFCF饮食增加了肝细胞中PPARγ的表达,罗格列酮在体内和体外进一步激活了HFCF喂养小鼠肝细胞中的PPARγ。肝细胞特异性 PPARγ 的丢失减少了 HFCF 诱导的雄性小鼠 NASH 的进展,并增加了 TZD 对肝外组织和非实质细胞的影响所带来的益处。RNAseq 和代谢组学表明,HFCF 饮食以肝细胞 PPARγ 依赖性方式促进炎症和纤维化,并与肝代谢失调有关。具体而言,肝细胞特异性 PPARγ 的丢失在蛋氨酸代谢的调节中起积极作用,这可能会减少 NASH 的进展。
结论
由于肝细胞 PPARγ 在 NASH 中的负面作用,抑制肝细胞中内源性 PPARγ 促进的机制可能代表一种提高 NAFLD 治疗效率的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号