Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-01-11 , DOI: 10.1016/j.bmc.2021.116014 Greta Klejborowska 1 , Alicja Urbaniak 2 , Ewa Maj 3 , Joanna Wietrzyk 3 , Mahshad Moshari 4 , Jordane Preto 5 , Jack A Tuszynski 6 , Timothy C Chambers 2 , Adam Huczyński 1
|
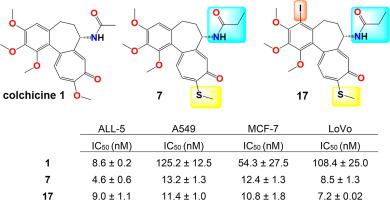
Colchicine is a plant alkaloid with a broad spectrum of biological and pharmacological properties. It has found application as an anti-inflammatory agent and also shows anticancer effects through its ability to destabilize microtubules by preventing tubulin dimers from polymerizing leading to mitotic death. However, adverse side effects have so far restricted its use in cancer therapy. This has led to renewed efforts to identify less toxic derivatives. In this article, we describe the synthesis of a set of novel double- and triple-modified colchicine derivatives. These derivatives were tested against primary acute lymphoblastic leukemia (ALL-5) cells and several established cancer cell lines including A549, MCF-7, LoVo and LoVo/DX. The novel derivatives were active in the low nanomolar range, with 7-deacetyl-10-thiocolchicine analogues more potent towards ALL-5 cells while 4-iodo-7-deacetyl-10-thiocolchicine analogues slightly more effective towards the LoVo cell line. Moreover, most of the synthesized compounds showed a favorable selectivity index (SI), particularly for ALL-5 and LoVo cell lines. Cell cycle analysis of the most potent molecules on ALL-5 and MCF-7 cell lines revealed contrasting effects, where M-phase arrest was observed in MCF-7 cells but not in ALL-5 cells. Molecular docking studies of all derivatives to the colchicine-binding site were performed and it was found that five of the derivatives showed strong β-tubulin binding energies, lower than −8.70 kcal/mol, while the binding energy calculated for colchicine is −8.09 kcal/mol. The present results indicate that 7-deacetyl-10-thiocolchicine and 4-iodo-7-deacetyl-10-thiocolchicine analogues constitute promising lead compounds as chemotherapy agents against several types of cancer.
中文翻译:

N-脱乙酰硫代秋水仙碱和4-碘-N-脱乙酰硫代秋水仙碱衍生物的合成、抗癌活性及分子对接研究
秋水仙碱是一种植物生物碱,具有广泛的生物学和药理特性。它已发现可用作抗炎剂,并通过阻止微管蛋白二聚体聚合导致有丝分裂死亡来破坏微管的能力,从而显示出抗癌作用。然而,迄今为止,不良副作用限制了其在癌症治疗中的应用。这导致重新努力鉴定毒性较小的衍生物。在本文中,我们描述了一组新型双和三修饰秋水仙碱衍生物的合成。这些衍生物针对原发性急性淋巴细胞白血病 (ALL-5) 细胞和几种已建立的癌细胞系进行了测试,包括 A549、MCF-7、LoVo 和 LoVo/DX。新型衍生物在低纳摩尔范围内具有活性,7-deacetyl-10-thiocolchicine 类似物对 ALL-5 细胞更有效,而 4-iodo-7-deacetyl-10-thiocolchicine 类似物对 LoVo 细胞系更有效。此外,大多数合成的化合物显示出良好的选择性指数 (SI),特别是对于 ALL-5 和 LoVo 细胞系。对 ALL-5 和 MCF-7 细胞系中最有效分子的细胞周期分析揭示了对比效应,其中 M 期阻滞在 MCF-7 细胞中观察到,但在 ALL-5 细胞中未观察到。对所有衍生物与秋水仙碱结合位点进行了分子对接研究,发现其中五种衍生物表现出很强的β-微管蛋白结合能,低于-8.70 kcal/mol,而秋水仙碱的结合能计算为-8.09 kcal /摩尔。


























 京公网安备 11010802027423号
京公网安备 11010802027423号