Journal of Advanced Research ( IF 10.7 ) Pub Date : 2021-01-08 , DOI: 10.1016/j.jare.2021.01.001 Nicol Parada 1, 2 , Alfonso Romero-Trujillo 1, 2 , Nicolás Georges 1, 2 , Francisca Alcayaga-Miranda 1, 2, 3, 4
|
Background
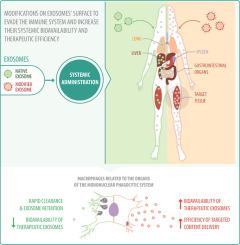
Even though exosome-based therapy has been shown to be able to control the progression of different pathologies, the data revealed by pharmacokinetic studies warn of the low residence time of exogenous exosomes in circulation that can hinder the clinical translation of therapeutic exosomes. The macrophages related to the organs of the mononuclear phagocytic system are responsible primarily for the rapid clearance and retention of exosomes, which strongly limits the amount of exosomal particles available to reach the target tissue, accumulate in it and release with high efficiency its therapeutic cargo in acceptor target cells to exert the desired biological effect.
Aim of review
Endowing exosomes with surface modifications to evade the immune system is a plausible strategy to contribute to the suppression of exosomal clearance and increase the efficiency of their targeted content delivery. Here, we summarize the current evidence about the mechanisms underlying the recognition and sequestration of therapeutic exosomes by phagocytic cells. Also, we propose different strategies to generate 'invisible' exosomes for the immune system, through the incorporation of different anti-phagocytic molecules on the exosomes’ surface that allow increasing the circulating half-life of therapeutic exosomes with the purpose to increase their bioavailability to reach the target tissue, transfer their therapeutic molecular cargo and improve their efficacy profile.
Key scientific concepts of review
Macrophage-mediated phagocytosis are the main responsible behind the short half-life in circulation of systemically injected exosomes, hindering their therapeutic effect. Exosomes ‘Camouflage Cloak’ strategy using antiphagocytic molecules can contribute to the inhibition of exosomal clearance, hence, increasing the on-target effect. Some candidate molecules that could exert an antiphagocytic role are CD47, CD24, CD44, CD31, β2M, PD-L1, App1, and DHMEQ. Pre- and post-isolation methods for exosome engineering are compatible with the loading of therapeutic cargo and the expression of antiphagocytic surface molecules.
中文翻译:

治疗性外泌体逃避吞噬作用的伪装策略
背景
尽管基于外泌体的治疗已被证明能够控制不同病理的进展,但药代动力学研究揭示的数据警告说,外源性外泌体在循环中的停留时间较短,这可能会阻碍治疗性外泌体的临床转化。与单核吞噬系统器官相关的巨噬细胞主要负责外泌体的快速清除和滞留,这极大地限制了可到达靶组织、在其中积累和高效释放其治疗货物的外泌体颗粒的数量。受体靶细胞发挥所需的生物学效应。
审查的目的
赋予外泌体表面修饰以逃避免疫系统是一种合理的策略,有助于抑制外泌体清除并提高其靶向内容传递的效率。在这里,我们总结了目前关于吞噬细胞识别和隔离治疗性外泌体的机制的证据。此外,我们提出了不同的策略来为免疫系统生成“隐形”外泌体,通过在外泌体表面掺入不同的抗吞噬分子,从而增加治疗性外泌体的循环半衰期,目的是增加它们的生物利用度。到达目标组织,转移其治疗分子货物并提高其疗效。
审查的关键科学概念
巨噬细胞介导的吞噬作用是全身注射外泌体循环半衰期短的主要原因,阻碍了它们的治疗效果。使用抗吞噬分子的外泌体“伪装斗篷”策略有助于抑制外泌体清除,从而增加靶向效应。一些可以发挥抗吞噬作用的候选分子是 CD47、CD24、CD44、CD31、β2M、PD-L1、App1 和 DHMEQ。外泌体工程的分离前和分离后方法与治疗货物的装载和抗吞噬细胞表面分子的表达相兼容。


























 京公网安备 11010802027423号
京公网安备 11010802027423号