Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2021-01-08 , DOI: 10.1016/j.bbamem.2021.183554 Chunxu Ni 1 , Xuyang Wang 1 , Jie Chen 1 , Su Xu 1 , Wenjing Ye 1 , Mei Hong 2
|
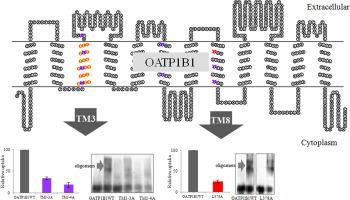
Organic anion transporting polypeptides (OATPs) are transmembrane proteins responsible for the uptake of a wide range of endogenous compounds and clinically important drugs. The liver-specific OATP1B1 serves crucial roles in the removal of many orally administered drugs. The proper function of the transporter hence is essential for the pharmacokinetics of various therapeutic agents. Membrane proteins tend to form oligomers that are important for their stability, targeting and/or interactions with the substrates. Previous study in our laboratory revealed that OATP1B1 may form homo-oligomers and that a GXXXG motif localized at transmembrane domain 8 (TM8) may affect its oligomerization. In the current study, three short-form leucine heptad repeats within the transmembrane domains of OATP1B1 were investigated. It was found that the disruption of leucine heptad repeats within TM3 dramatically reduced the uptake function and protein-protein association of OATP1B1; while within TM8, only L378 is essential for the function of OATP1B1 and alanine replacement of L378 exhibited no effect on the oligomerization. The fragmental expression of TM3 interfered with the association of OATP1B1 homo-oligomers as well as its association with OATP1B3, which is also selectively expressed at human hepatocytes, suggesting that the region may be shared by both transporters for their protein-protein interactions.
中文翻译:

跨膜结构域中的亮氨酸七肽基序影响人类有机阴离子转运多肽1B1的功能和寡聚。
有机阴离子转运多肽(OATP)是跨膜蛋白,负责摄取多种内源性化合物和临床上重要的药物。肝脏特异性OATP1B1在去除许多口服药物中起着至关重要的作用。因此,转运蛋白的适当功能对于各种治疗剂的药代动力学至关重要。膜蛋白趋于形成寡聚物,这些寡聚物对于它们的稳定性,靶向性和/或与底物的相互作用很重要。我们实验室中的先前研究表明,OATP1B1可能形成同源寡聚体,而位于跨膜结构域8(TM8)的GXXXG基序可能会影响其寡聚化。在当前的研究中,调查了OATP1B1跨膜域内的三个短形式亮氨酸七肽重复序列。发现TM3内亮氨酸七肽重复序列的破坏显着降低了OATP1B1的吸收功能和蛋白-蛋白缔合。而在TM8中,只有L378对于OATP1B1的功能是必不可少的,而L378的丙氨酸替代对寡聚化没有影响。TM3的片段表达干扰了OATP1B1同源寡聚体的结合及其与OATP1B3的结合,OATP1B3也选择性地在人肝细胞中表达,表明该区域可能由两个转运蛋白共享,因为它们之间存在蛋白质-蛋白质相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号