当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Membrane Interactions of α-Synuclein Probed by Neutrons and Photons
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2021-01-08 , DOI: 10.1021/acs.accounts.0c00453 Upneet Kaur 1 , Jennifer C Lee 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2021-01-08 , DOI: 10.1021/acs.accounts.0c00453 Upneet Kaur 1 , Jennifer C Lee 1
Affiliation
|
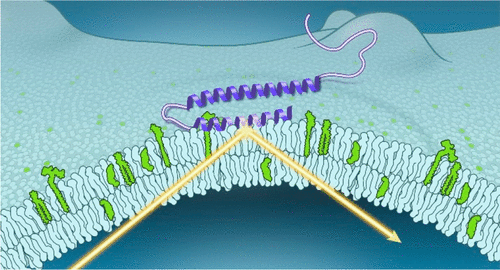
α-Synuclein (α-syn) is a key protein in the etiology of Parkinson’s disease. In a disease state, α-syn accumulates as insoluble amyloid fibrils enriched in β-sheet structure. However, in its functional state, α-syn adopts an amphipathic helix upon membrane association and plays a role in synaptic vesicle docking, fusion, and clustering. In this Account, we describe our contributions made in the past decade toward developing a molecular understanding of α-syn membrane interactions, which are crucial for function and have pathological implications. Three topics are covered: α-syn membrane binding probed by neutron reflectometry (NR), the effects of membrane on α-syn amyloid formation, and interactions of α-syn with cellular membranes.
中文翻译:

中子和光子探测的α-突触核蛋白的膜相互作用
α-突触核蛋白 (α-syn) 是帕金森病病因学中的关键蛋白。在疾病状态下,α-syn 积累为富含 β-折叠结构的不溶性淀粉样蛋白原纤维。然而,在其功能状态下,α-syn 在膜结合时采用两亲螺旋,并在突触小泡对接、融合和聚集中发挥作用。在这个帐户中,我们描述了我们在过去十年中为发展对 α-syn 膜相互作用的分子理解所做的贡献,这对功能至关重要并具有病理学意义。涵盖三个主题:通过中子反射法 (NR) 探测的 α-syn 膜结合、膜对 α-syn 淀粉样蛋白形成的影响以及 α-syn 与细胞膜的相互作用。
更新日期:2021-01-19
中文翻译:

中子和光子探测的α-突触核蛋白的膜相互作用
α-突触核蛋白 (α-syn) 是帕金森病病因学中的关键蛋白。在疾病状态下,α-syn 积累为富含 β-折叠结构的不溶性淀粉样蛋白原纤维。然而,在其功能状态下,α-syn 在膜结合时采用两亲螺旋,并在突触小泡对接、融合和聚集中发挥作用。在这个帐户中,我们描述了我们在过去十年中为发展对 α-syn 膜相互作用的分子理解所做的贡献,这对功能至关重要并具有病理学意义。涵盖三个主题:通过中子反射法 (NR) 探测的 α-syn 膜结合、膜对 α-syn 淀粉样蛋白形成的影响以及 α-syn 与细胞膜的相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号