International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2021-01-08 , DOI: 10.1016/j.ijms.2021.116524 Yang Du , Fengjiao Zhao , Junpeng Xing , Meng Cui , Zhiqiang Liu
|
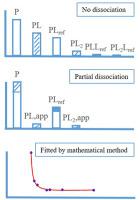
The investigations of noncovalent interaction between proteins (P) and small ligands (L) have played an important role for drug discovery and therapy. Electrospray ionization mass spectrometry (ESI-MS) has been typically applied to analysis of P-L interactions. However, some labile complexes are problematic for determination of association constants (Ka) by ESI-MS due to their in-source dissociation. Here, a strategy based on native MS for analysis of the labile complexes was developed. The reference ligand (Lref) with specific binding to P and a nonspecific binding ligand were employed. The equations for Ka calculation of labile complexes were developed. According to mathematical fitting curves, the Ka values of partially dissociated lysozyme-icariin complex and completely dissociated lysozyme-berberine complex were obtained. The obtained Ka values were consistent with those by fluorescence method. This strategy can be applied to the labile complexes of other small ligands to protein.
中文翻译:

天然电喷雾电离质谱研究不稳定的蛋白质-配体相互作用
蛋白质(P)和小配体(L)之间非共价相互作用的研究对于药物发现和治疗起了重要作用。电喷雾电离质谱(ESI-MS)通常已用于分析PL相互作用。然而,一些不稳定的络合物是用于测定缔合常数(K的问题一)通过ESI-MS由于它们在源离解。在这里,开发了一种基于天然MS的分析不稳定复合物的策略。使用对P具有特异性结合的参考配体(L ref)和非特异性结合配体。开发了用于计算不稳定配合物的K a的方程。根据数学拟合曲线,K a获得了部分解离的溶菌酶-大豆素复合物和完全解离的溶菌酶-小ber碱复合物的值。所得到的K a值与荧光法一致。该策略可应用于其他小的配体与蛋白质的不稳定复合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号