当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ruthenium-catalysed C–H/C–N bond activation: facile access to isoindolinones
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0qo01406k Xiao-Qiang Hu 1, 2, 3, 4, 5 , Ye-Xing Hou 1, 2, 3, 4, 5 , Zi-Kui Liu 1, 2, 3, 4, 5 , Yang Gao 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0qo01406k Xiao-Qiang Hu 1, 2, 3, 4, 5 , Ye-Xing Hou 1, 2, 3, 4, 5 , Zi-Kui Liu 1, 2, 3, 4, 5 , Yang Gao 5, 6, 7, 8
Affiliation
|
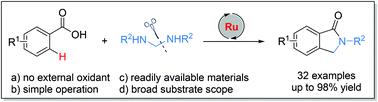
A facile ruthenium-catalysed C–H/C–N bond activation and the subsequent annulation of readily available benzoic acids with in situ generated formaldimines are reported. The choice of solvent and base is critical for this reaction. This protocol allows the efficient synthesis of a wide range of biologically important isoindolinones in useful to excellent yields without the use of any external oxidants. The late-stage modification of bioactive acids such as adapalene, bexarotene and flufenamic acid demonstrated the synthetic utility of this methodology.
中文翻译:

钌催化的C–H / C–N键活化:容易获得异吲哚啉酮
据报道,钌催化的C–H / C–N键很容易活化,随后随处可见的苯甲酸与原位生成的福尔马明环化。溶剂和碱的选择对于该反应至关重要。该方案可有效合成范围广泛的生物学上重要的异吲哚啉酮,以高产率获得而无需使用任何外部氧化剂。生物活性酸(如阿达帕林,贝沙罗汀和氟苯那酸)的后期修饰证明了该方法的合成效用。
更新日期:2021-01-07
中文翻译:

钌催化的C–H / C–N键活化:容易获得异吲哚啉酮
据报道,钌催化的C–H / C–N键很容易活化,随后随处可见的苯甲酸与原位生成的福尔马明环化。溶剂和碱的选择对于该反应至关重要。该方案可有效合成范围广泛的生物学上重要的异吲哚啉酮,以高产率获得而无需使用任何外部氧化剂。生物活性酸(如阿达帕林,贝沙罗汀和氟苯那酸)的后期修饰证明了该方法的合成效用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号