当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Transformations of Sulfonium Salts via C-S Bond Activation
The Chemical Record ( IF 6.6 ) Pub Date : 2021-01-07 , DOI: 10.1002/tcr.202000172 Hideki Yorimitsu 1
The Chemical Record ( IF 6.6 ) Pub Date : 2021-01-07 , DOI: 10.1002/tcr.202000172 Hideki Yorimitsu 1
Affiliation
|
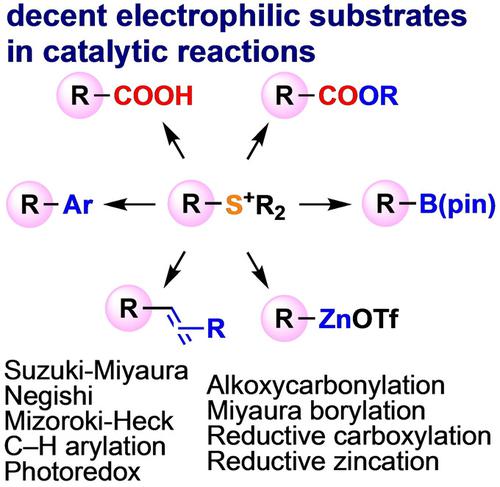
After the 20-year dormant period since Liebeskind's seminal report, triorganosulfonium salts have now proved to be reliably useful surrogates of or complement to organic halides in a variety of transition-metal-catalyzed transformations. By necessity, my group developed Suzuki-Miyaura ring-opening arylation of sulfonium salts of dibenzothiophenes with tetraarylborates and Pd-catalyzed intramolecular C−H/C−S coupling. Following this starting point and experiencing the decent reactivity of sulfonium salts, in order to maximize the synthetic utility of organosulfur compounds, we successfully applied readily available sulfonium salts to Ni-catalyzed coupling with arylzinc reagents, Pd- or photoredox-catalyzed Mizoroki-Heck reaction, Pd-catalyzed alkoxycarbonylation, Pd-catalyzed Miyaura borylation, Ni-catalyzed reductive carboxylation, and Ni-catalyzed zincation. I sincerely hope this Personal Account will facilitate further development of reactions of triorganosulfonium salts which are difficult to achieve using conventional organic (pseudo)halides.
中文翻译:

通过 CS 键活化催化转化锍盐
在 Liebeskind 的开创性报告之后的 20 年休眠期之后,三有机锍盐现在已被证明是各种过渡金属催化转化中有机卤化物的可靠替代物或补充物。根据需要,我的团队开发了二苯并噻吩锍盐与四芳基硼酸盐的 Suzuki-Miyaura 开环芳基化和 Pd 催化的分子内 C-H/C-S 偶联。遵循这一出发点并体验锍盐的良好反应性,为了最大限度地提高有机硫化合物的合成效用,我们成功地将现成的锍盐应用于 Ni 催化与芳基锌试剂偶联、Pd 或光氧化还原催化的 Mizoroki-Heck 反应, 钯催化的烷氧基羰基化, 钯催化的宫浦硼化, 镍催化的还原羧化, 和镍催化的锌化。我真诚地希望这个个人账户能够促进三有机锍盐反应的进一步发展,而这些反应是使用传统的有机(伪)卤化物难以实现的。
更新日期:2021-01-07
中文翻译:

通过 CS 键活化催化转化锍盐
在 Liebeskind 的开创性报告之后的 20 年休眠期之后,三有机锍盐现在已被证明是各种过渡金属催化转化中有机卤化物的可靠替代物或补充物。根据需要,我的团队开发了二苯并噻吩锍盐与四芳基硼酸盐的 Suzuki-Miyaura 开环芳基化和 Pd 催化的分子内 C-H/C-S 偶联。遵循这一出发点并体验锍盐的良好反应性,为了最大限度地提高有机硫化合物的合成效用,我们成功地将现成的锍盐应用于 Ni 催化与芳基锌试剂偶联、Pd 或光氧化还原催化的 Mizoroki-Heck 反应, 钯催化的烷氧基羰基化, 钯催化的宫浦硼化, 镍催化的还原羧化, 和镍催化的锌化。我真诚地希望这个个人账户能够促进三有机锍盐反应的进一步发展,而这些反应是使用传统的有机(伪)卤化物难以实现的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号