当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substrate binding in the bile acid transporter ASBTYf from Yersinia frederiksenii
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-01-06 , DOI: 10.1107/s2059798320015004 Xiaodong Wang 1 , Ying Lyu 1 , Yujia Ji 1 , Ziyi Sun 1 , Xiaoming Zhou 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-01-06 , DOI: 10.1107/s2059798320015004 Xiaodong Wang 1 , Ying Lyu 1 , Yujia Ji 1 , Ziyi Sun 1 , Xiaoming Zhou 1
Affiliation
|
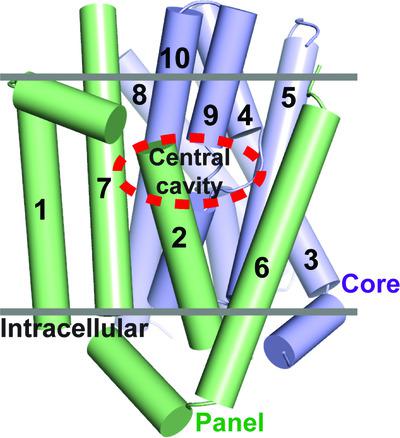
Apical sodium‐dependent bile acid transporter (ASBT) retrieves bile acids from the small intestine and plays a pivotal role in enterohepatic circulation. Currently, high‐resolution structures are available for two bacterial ASBT homologs (ASBTNM from Neisseria meningitides and ASBTYf from Yersinia frederiksenii), from which an elevator‐style alternating‐access mechanism has been proposed for substrate transport. A key concept in this model is that the substrate binds to the central cavity of the transporter so that the elevator‐like motion can expose the bound substrate alternatingly to either side of the membrane during a transport cycle. However, no structure of an ASBT has been solved with a substrate bound in its central cavity, so how a substrate binds to ASBT remains to be defined. In this study, molecular docking, structure determination and functional analysis were combined to define and validate the details of substrate binding in ASBTYf. The findings provide coherent evidence to provide a clearer picture of how the substrate binds in the central cavity of ASBTYf that fits the alternating‐access model.
中文翻译:

来自弗雷德里克森耶尔森氏菌的胆汁酸转运蛋白 ASBTYf 中的底物结合
顶端钠依赖性胆汁酸转运蛋白 (ASBT) 从小肠中回收胆汁酸,并在肠肝循环中发挥关键作用。目前,高分辨率结构可用于两种细菌 ASBT 同源物(来自脑膜炎奈瑟菌的ASBT NM和来自耶尔森氏菌的ASBT Yf)),从中提出了一种用于基板传输的电梯式交替访问机制。该模型中的一个关键概念是,基材与转运体的中心腔结合,因此类似电梯的运动可以在运输周期中交替地将结合的基材暴露在膜的任一侧。然而,ASBT 的结构尚未在其中心腔中结合底物得到解决,因此底物如何与 ASBT 结合仍有待定义。在这项研究中,结合分子对接、结构确定和功能分析来定义和验证 ASBT Yf中底物结合的细节。这些发现提供了连贯的证据,可以更清楚地了解底物如何在 ASBT Yf的中心腔中结合 这符合交替访问模型。
更新日期:2021-01-06
中文翻译:

来自弗雷德里克森耶尔森氏菌的胆汁酸转运蛋白 ASBTYf 中的底物结合
顶端钠依赖性胆汁酸转运蛋白 (ASBT) 从小肠中回收胆汁酸,并在肠肝循环中发挥关键作用。目前,高分辨率结构可用于两种细菌 ASBT 同源物(来自脑膜炎奈瑟菌的ASBT NM和来自耶尔森氏菌的ASBT Yf)),从中提出了一种用于基板传输的电梯式交替访问机制。该模型中的一个关键概念是,基材与转运体的中心腔结合,因此类似电梯的运动可以在运输周期中交替地将结合的基材暴露在膜的任一侧。然而,ASBT 的结构尚未在其中心腔中结合底物得到解决,因此底物如何与 ASBT 结合仍有待定义。在这项研究中,结合分子对接、结构确定和功能分析来定义和验证 ASBT Yf中底物结合的细节。这些发现提供了连贯的证据,可以更清楚地了解底物如何在 ASBT Yf的中心腔中结合 这符合交替访问模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号