Reactive & Functional Polymers ( IF 5.1 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.reactfunctpolym.2021.104809 Zhengchi Yang , Lihua Liu , Siyan Liu , Gang Su , Xing Liu , Anping Tang , Jianrong Xue , Mengxiang Zeng
|
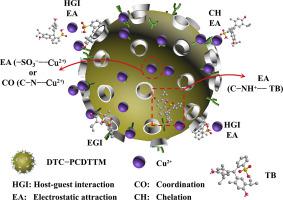
Novel dithiocarbamate (DTC)-modified crosslinked poly(β−cyclodextrin-co-triethylenetetramine) microspheres (DTC-PCDTTM) possessing a particle size distribution of 141 μm with a span value of 0.65 were fabricated and evaluated by Cu2+−MO/TB binary-component systems. DTC-PCDTTM shows excellent simultaneous adsorption performance for Cu2+ and methyl orange (MO) or thymol blue (TB) at a DTC graft ratio of 0.21; it also exhibits eminent regeneration and recyclability. The adsorption isotherm data of DTC-PCDTTM for Cu2+ and MO or TB in Cu2+−MO/TB binary-component systems can be described by the Freundlich and Langmuir models, respectively. The adsorption kinetic data of Cu2+, MO, and TB by DTC-PCDTTM are related to the pseudo-second-order kinetic model. The adsorption is a process of spontaneous endothermic entropy increment. The coordination, electrostatic attraction, and the host−guest interaction of β−cD moieties with contaminants are the primary mechanisms for Cu2+, MO, and TB adsorption. In the Cu2+−MO/TB binary-component systems, competitive adsorption is effectively avoided, and Cu2+ shows good synergistic effects with MO and TB.
中文翻译:

二硫代氨基甲酸酯改性的交联聚(β-环糊精-三亚乙基四胺)微球的合成,用于同时高效去除废水中的Cu 2+和甲基橙/百里酚蓝
制备了具有141μm粒径分布,跨度值为0.65的新型二硫代氨基甲酸酯(DTC)改性的交联聚(β-环糊精-三亚乙基四胺)微球(DTC-PCDTTM)并通过Cu 2+ -MO / TB进行了评估二进制组件系统。DTC-PCDTTM在0.21的DTC接枝率下对Cu 2+和甲基橙(MO)或百里酚蓝(TB)表现出优异的同时吸附性能;它还具有出色的再生和可回收性。Cu 2+ -MO / TB二元体系中DTC-PCDTTM对Cu 2+和MO或TB的吸附等温线数据可以分别用Freundlich和Langmuir模型描述。Cu 2+的吸附动力学数据DTC-PCDTTM的,MO和TB与伪二级动力学模型有关。吸附是自发吸热熵增加的过程。β-cD部分与污染物的配位,静电吸引和主客体相互作用是Cu 2 +,MO和TB吸附的主要机理。在Cu 2+ -MO / TB二元体系中,有效地避免了竞争性吸附,Cu 2+与MO和TB具有良好的协同作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号