Molecular Catalysis ( IF 4.6 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.mcat.2020.111333 Alaa H. Alminshid , Mohammed N. Abbas , Hayder A. Alalwan , Abbas J. Sultan , Mohammed A. Kadhom
|
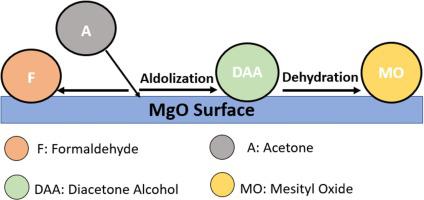
Acetone adsorption and catalytic interactions with a MgO nanoparticle surface was studied using in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) at room temperature. Acetone showed adsorption bands at 2965, 1717, 1597, 1422, 1415, 1367, 1238, 1229, and 1204 cm−1. The MgO was found to be a good catalyst for the activation of the aldol condensation reaction of acetone to form di-acetonealcohol (DAA). The OH group role on the MgO surface was found to be a suppression agent to the further dehydration of DAA to mesityl oxide. This is probably because the OH group blocks the active sites on the MgO surfaces that adsorbed the water formed from the dehydration of DAA. This conclusion was confirmed by identifying the basic site concentrations on MgO surface using DRIFTS and TPD studies of CO2 adsorption/desorption on MgO nanoparticles surface. The high-strength basic sites which are required to dehydrate DDA to mesityl oxide was found to represent only 17.5 % of the total basic site concentration, while the low-strength basic sites resulted from hydroxyl surface group represent 22.5 %. On the other hand, the acetone interaction with the MgO surfaces produced several components such as formaldehyde, acetate, ethoxide, as well as other carbonate species such as bicarbonate, bidentate carbonate, and monodentate carbonate. The results of the present investigation supply important essential insights into catalytic reactions resulted from acetone adsorption on MgO nanoparticle surfaces.
中文翻译:

丙酮在MgO纳米颗粒表面上的醛醇缩合反应:原位漂移研究
在室温下使用原位漫反射红外傅里叶变换光谱仪(DRIFTS)研究了丙酮与MgO纳米颗粒表面的吸附和催化相互作用。丙酮在2965、1717、1597、1422、1415、1367、1238、1229和1204 cm -1处显示出吸附带。发现MgO是激活丙酮的醛醇缩合反应以形成二丙酮醇(DAA)的良好催化剂。发现在MgO表面上的OH基团作用是DAA进一步脱水成异氰酸酯的抑制剂。这可能是因为OH基团阻塞了吸附由DAA脱水形成的水的MgO表面上的活性位。通过使用DRIFTS和TPD研究CO 2来确定MgO表面的基本位点浓度,从而证实了这一结论。在氧化镁纳米颗粒表面的吸附/解吸。发现将DDA脱水为氧化异丁烯所需的高强度碱性位点仅占总碱性位点浓度的17.5%,而羟基表面基团导致的低强度碱性位点占22.5%。另一方面,丙酮与MgO表面的相互作用产生了几种组分,例如甲醛,乙酸盐,乙醇盐,以及其他碳酸盐种类,例如碳酸氢盐,双齿碳酸盐和单齿碳酸盐。本研究的结果为丙酮吸附在MgO纳米颗粒表面上引起的催化反应提供了重要的基本见识。

























 京公网安备 11010802027423号
京公网安备 11010802027423号