Biomaterials Advances ( IF 7.9 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.msec.2020.111854 Barbara Blanco-Fernandez , Irene Cano-Torres , Cristina Garrido , Gerard Rubi-Sans , Lourdes Sanchez-Cid , Marta Guerra-Rebollo , Nuria Rubio , Jeronimo Blanco , Soledad Perez-Amodio , Miguel A. Mateos-Timoneda , Elisabeth Engel
|
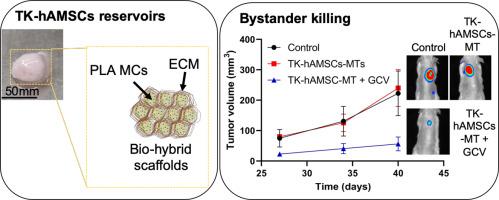
Thymidine kinase expressing human adipose mesenchymal stem cells (TK-hAMSCs) in combination with ganciclovir (GCV) are an effective platform for antitumor bystander therapy in mice models. However, this strategy requires multiple TK-hAMSCs administrations and a substantial number of cells. Therefore, for clinical translation, it is necessary to find a biocompatible scaffold providing TK-hAMSCs retention in the implantation site against their rapid wash-out. We have developed a microtissue (MT) composed by TKhAMSCs and a scaffold made of polylactic acid microparticles and cell-derived extracellular matrix deposited by hAMSCs. The efficacy of these MTs as vehicles for TK-hAMSCs/GCV bystander therapy was evaluated in a rodent model of human prostate cancer. Subcutaneously implanted MTs were integrated in the surrounding tissue, allowing neovascularization and maintenance of TK-hAMSCs viability. Furthermore, MTs implanted beside tumors allowed TK-hAMSCs migration towards tumor cells and, after GCV administration, inhibited tumor growth. These results indicate that TK-hAMSCs-MTs are promising cell reservoirs for clinical use of therapeutic MSCs in bystander therapies.
中文翻译:

工程化的微组织,用于癌症旁观者治疗
表达胸苷激酶的人脂肪间充质干细胞(TK-hAMSC)与更昔洛韦(GCV)的结合是小鼠模型中抗肿瘤旁观者治疗的有效平台。但是,此策略需要多次TK-hAMSC施用和大量细胞。因此,对于临床翻译,有必要找到一种生物相容性支架,该支架可将TK-hAMSCs保留在植入位点,以防止其快速洗去。我们已经开发了由TKhAMSC组成的微组织(MT),以及由hAMSC沉积的聚乳酸微粒和细胞衍生的细胞外基质制成的支架。在人类前列腺癌的啮齿动物模型中评估了这些MT作为TK-hAMSCs / GCV旁观者治疗的载体的功效。皮下植入的MT整合在周围组织中,可以使新血管形成并维持TK-hAMSC的生存能力。此外,在肿瘤旁植入的MTs使TK-hAMSCs向肿瘤细胞迁移,并在施用GCV后抑制了肿瘤的生长。这些结果表明,TK-hAMSCs-MTs是有希望的细胞储库,用于临床治疗性MSCs在旁观者疗法中的临床应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号