Chemical Geology ( IF 3.9 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.chemgeo.2020.120034 A.M. Trueman , R.W. Fitzpatrick , L.M. Mosley , M.J. McLaughlin
|
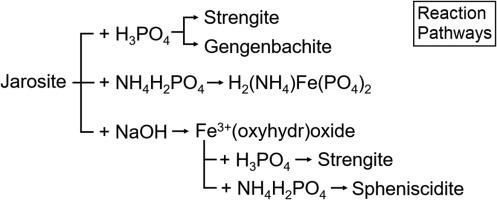
Jarosite (KFe3(SO4)2(OH)6) is a common secondary reaction product of iron sulfide oxidation and can pose a considerable risk to soil and water quality. The overarching objective of this study was to explore the possibility of employing passivation-based treatments to mitigate the risks associated with jarosite-rich materials by investigating the alteration of pedogenic jarosite to a relatively benign, sparingly-soluble mineral such as goethite or strengite. Samples of jarositic phytotubules (from an acid sulfate soil with sulfuric material) were treated with: (i) alkaline solutions with a view to produce ferric (oxyhydr)oxide; (ii) phosphate solutions with a view to produce ferric phosphate; and (iii) alkaline solutions, followed by phosphate solutions, with a view to produce ferric phosphate. These treatments were conducted at both ambient (25°C) and elevated temperatures (80°C) to explore the effects of temperature on the extent of jarosite alteration. Under alkaline conditions, jarosite readily decomposed to yield poorly-crystalline ferric (oxyhydr)oxides (e.g. two-line ferrihydrite), regardless of temperature. These ferric (oxyhydr)oxides readily reacted with phosphoric acid to yield ferric phosphate; and with monoammonium phosphate (MAP) to yield spheniscidite ((NH4,K)(Fe,Al)2(PO4)2(OH).2H2O). Jarosite reacted with phosphoric acid to produce strengite or phosphosiderite (depending on the reaction temperature) and, to a lesser extent, gengenbachite (KFe3(HPO4)4(H2PO4)2.6H2O). Similarly, jarosite reacted with MAP to produce hydrogen ammonium ferric phosphate (H2(NH4)Fe(PO4)2). The direct alteration of jarosite to ferric phosphate appears to be very limited under ambient conditions. However, this study predominantly focuses on changes to the bulk chemical composition of jarosite. Consequently, further investigation (e.g. SEM analysis) is recommended to explore subtle changes in surface chemistry and the related effects on jarosite reactivity. Column perfusion experiments were also conducted to study the release of key elements during the dissolution of NaOH- and MAP-treated jarosite. The results suggest that these treatments do not considerably lower the risks to soil and water quality posed by jarosite dissolution. The practicality of the treatments is discussed, including the potential negative side effects. Further investigation is required to determine if these, or alternative, passivation treatments could be employed in the field to effectively mitigate the environmental risk associated with jarosite.
中文翻译:

探索基于钝化的酸性硫酸盐土壤中黄钾铁矾的处理方法
黄铁矿(KFe 3(SO 4)2(OH)6)是硫化铁氧化的常见二级反应产物,可能对土壤和水质构成相当大的风险。这项研究的总体目标是通过研究将成岩黄钾铁矾转变为相对温和,微溶的针铁矿或针铁矿,来探索采用钝化处理来降低与富含黄钾铁矾的材料相关的风险的可能性。(i)碱性溶液以产生三氧化二铁(碱土金属的氧化物);(ii)为了产生磷酸铁的磷酸盐溶液;(iii)碱性溶液,然后是磷酸盐溶液,以生产磷酸铁。这些处理均在环境温度(25° C)和高温(80° C),以探索温度对黄铁矿蚀变程度的影响。在碱性条件下,无论温度如何,黄钾铁矾都容易分解,生成结晶度较差的三氧化二铁(羟基氧化铁)。这些三氧化二铁易于与磷酸反应生成磷酸铁。并与磷酸一铵(MAP)一起生成亚长晶石((NH 4,K)(Fe,Al)2(PO 4)2(OH).2H 2 O)。黄铁矿与磷酸反应生成硬铁矿或亚磷酸铁矿(取决于反应温度),并在较小程度上生成长晶石(KFe 3(HPO 4)4(H 2 PO 4)2 .6H 2 O)。同样,黄铁矿与MAP反应生成磷酸氢铁铵(H 2(NH 4)Fe(PO 4)2)。在环境条件下,黄铁矿直接转变为磷酸铁似乎非常有限。但是,这项研究主要集中在改变黄钾铁矾的整体化学组成上。因此,建议进一步研究(例如SEM分析)以探索表面化学的细微变化以及对黄铁矿反应性的相关影响。还进行了色谱柱灌注实验,以研究NaOH和MAP处理的黄铁矿溶解过程中关键元素的释放。结果表明,这些处理并未显着降低黄钾铁矾溶解对土壤和水质造成的风险。讨论了治疗的实用性,包括潜在的负面副作用。需要进一步调查以确定是否这些或替代方案

























 京公网安备 11010802027423号
京公网安备 11010802027423号