Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.bmc.2021.115998 Alice Pomeislová 1 , Miroslav Otmar 2 , Petra Rubešová 3 , Jakub Benýšek 3 , Marika Matoušová 3 , Helena Mertlíková-Kaiserová 3 , Radek Pohl 3 , Lenka Poštová Slavětínská 3 , Karel Pomeisl 4 , Marcela Krečmerová 3
|
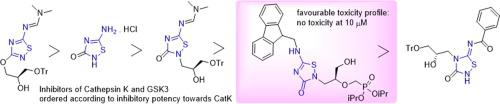
In analogy to antiviral acyclic nucleoside phosphonates, a series of 5-amino-3-oxo-1,2,4-thiadiazol-3(2H)-ones bearing a 2-phosphonomethoxyethyl (PME) or 3-hydroxy-2-(phosphonomethoxy)propyl (HPMP) group at the position 2 of the heterocyclic moiety has been synthesized. Diisopropyl esters of PME- and HPMP-amines have been converted to the N-substituted ureas and then reacted with benzoyl, ethoxycarbonyl, and Fmoc isothiocyanates to give the corresponding thiobiurets, which were oxidatively cyclized to diisopropyl esters of 5-amino-3-oxo-2-PME- or 2-HPMP- 1,2,4-thiadiazol-3(2H)-ones. The phosphonate ester groups were cleaved with bromotrimethylsilane, yielding N5-protected phosphonic acids. The subsequent attempts to remove the protecting group from N5 under alkaline conditions resulted in the cleavage of the 1,2,4-thiadiazole ring. Similarly, compounds with a previously unprotected 5-amino-1,2,4-thiadiazolone base moiety were stable only in the form of phosphonate esters. The series of twenty-one newly prepared 1,2,4-thiadiazol-3(2H)-ones were explored as potential inhibitors of cysteine-dependent enzymes – human cathepsin K (CatK) and glycogen synthase kinase 3β (GSK-3β). Several compounds exhibited an inhibitory activity toward both enzymes in the low micromolar range. The inhibitory potency of some of them toward GSK-3β was similar to that of the thiadiazole GSK-3β inhibitor tideglusib, whereas others exhibited more favorable toxicity profile while retaining good inhibitory activity.
中文翻译:

1,2,4-噻二唑无环核苷膦酸酯作为半胱氨酸依赖性酶组织蛋白酶 K 和 GSK-3β 的抑制剂
类似于抗病毒非环状核苷膦酸酯,一系列5-氨基-3-氧代-1,2,4-噻二唑-3(2 ħ) -酮轴承2- phosphonomethoxyethyl(PME)或3-羟基-2-(已合成杂环部分 2 位的膦酰基甲氧基)丙基(HPMP)基团。PME-和HPMP-胺的二异丙酯已经转化为N-取代的脲,然后与苯甲酰基、乙氧基羰基和Fmoc异硫氰酸酯反应得到相应的硫代缩二脲,它们被氧化环化为5-氨基-3-氧代的二异丙酯-2-PME- 或 2-HPMP- 1,2,4-thiadiazol-3(2 H )-ones。膦酸酯基团用溴代三甲基硅烷裂解,产生N 5-受保护的膦酸。随后在碱性条件下从N 5去除保护基团的尝试导致 1,2,4-噻二唑环断裂。类似地,具有先前未保护的 5-氨基-1,2,4-噻二唑酮碱部分的化合物仅以膦酸酯的形式稳定。新制备的 1,2,4-噻二唑-3(2 H)-ones 被探索作为半胱氨酸依赖性酶的潜在抑制剂 - 人组织蛋白酶 K (CatK) 和糖原合酶激酶 3β (GSK-3β)。几种化合物在低微摩尔范围内对两种酶都表现出抑制活性。其中一些对 GSK-3β 的抑制效力与噻二唑 GSK-3β 抑制剂tideglusib 的抑制效力相似,而另一些则表现出更有利的毒性特征,同时保持良好的抑制活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号