当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aqueous solubility of Pb at equilibrium with hydroxypyromorphite over a range of phosphate concentrations
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-12-23 , DOI: 10.1039/d0em00430h Yuting Zhou 1, 2, 3, 4 , Xinxin Li 4, 5, 6, 7 , Murray B. McBride 8, 9, 10, 11
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-12-23 , DOI: 10.1039/d0em00430h Yuting Zhou 1, 2, 3, 4 , Xinxin Li 4, 5, 6, 7 , Murray B. McBride 8, 9, 10, 11
Affiliation
|
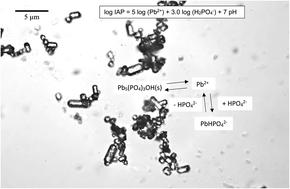
Hydroxypyromorphite (HPM) is a low-solubility Pb phosphate mineral that has the potential to limit solubility and bioavailability of Pb in soils and water. Because of reported uncertainty regarding the solubility product of this important mineral, we re-evaluated the solubility of Pb and activity of the free Pb2+ ion in aqueous suspensions of microcrystalline HPM equilibrated up to 30 days over a wide range of added soluble phosphate. A small addition of phosphate (0.1 mM) reduced Pb solubility as measured by ICP-OES, but greater phosphate additions (up to 50 mM) had no further effect in lowering HPM solubility. However, free Pb2+ ion activity measured by ion-selective electrode progressively decreased from about 10−6.5 with no added phosphate to 10−9 as soluble phosphate was increased. The effect of soluble phosphate in lowering Pb2+ activity is attributed to inhibited dissolution of HPM as well as increased Pb2+-phosphate ion pair formation in solution at higher solution concentrations of phosphate. Measurement of the ion activity products (IAP) of the solutions at equilibrium with HPM gave highly variable IAP values that were sensitive to pH and were generally not consistent with the reported solubility product of this mineral. The high variability of the IAPs for solutions with variable pH and phosphate concentrations indicates that dissolution–precipitation reactions of HPM are not described by a constant solubility product at equilibrium, possibly because of the incongruent dissolution behavior of this mineral at near-neutral pH.
中文翻译:

在一定范围的磷酸盐浓度下,Pb与羟基亚铬铁矿平衡时的水溶性
羟基焦晶石(HPM)是一种低溶解度的Pb磷酸盐矿物,具有限制Pb在土壤和水中的溶解度和生物利用度的潜力。由于所报告的关于这种重要矿物的溶解度产品的不确定性,我们重新评估了Pb的溶解度和游离Pb 2+离子在各种添加的可溶性磷酸盐中平衡长达30天的微晶HPM的水悬浮液中的活性。通过ICP-OES测量,少量添加磷酸盐(0.1 mM)降低了Pb的溶解度,但是添加大量磷酸盐(最高50 mM)并没有进一步降低HPM的溶解度。但是,通过离子选择电极测得的自由Pb 2+离子活性从未添加磷酸盐的约10 -6.5逐渐降低至10-9随着可溶性磷酸盐的增加而增加。可溶性磷酸盐降低Pb 2+活性的作用归因于HPM的溶解抑制以及Pb 2+的增加在较高的磷酸盐溶液浓度下在溶液中形成-磷酸盐离子对。用HPM平衡溶液的离子活性产物(IAP)的测量给出了高度可变的IAP值,该值对pH敏感,通常与所报道的这种矿物的溶解度产物不一致。对于具有可变pH和磷酸盐浓度的溶液,IAP的高度可变性表明HPM的溶解-沉淀反应不能用平衡状态下的恒定溶解度产物来描述,这可能是由于该矿物在接近中性pH值下的溶解行为不一致所致。
更新日期:2021-01-05
中文翻译:

在一定范围的磷酸盐浓度下,Pb与羟基亚铬铁矿平衡时的水溶性
羟基焦晶石(HPM)是一种低溶解度的Pb磷酸盐矿物,具有限制Pb在土壤和水中的溶解度和生物利用度的潜力。由于所报告的关于这种重要矿物的溶解度产品的不确定性,我们重新评估了Pb的溶解度和游离Pb 2+离子在各种添加的可溶性磷酸盐中平衡长达30天的微晶HPM的水悬浮液中的活性。通过ICP-OES测量,少量添加磷酸盐(0.1 mM)降低了Pb的溶解度,但是添加大量磷酸盐(最高50 mM)并没有进一步降低HPM的溶解度。但是,通过离子选择电极测得的自由Pb 2+离子活性从未添加磷酸盐的约10 -6.5逐渐降低至10-9随着可溶性磷酸盐的增加而增加。可溶性磷酸盐降低Pb 2+活性的作用归因于HPM的溶解抑制以及Pb 2+的增加在较高的磷酸盐溶液浓度下在溶液中形成-磷酸盐离子对。用HPM平衡溶液的离子活性产物(IAP)的测量给出了高度可变的IAP值,该值对pH敏感,通常与所报道的这种矿物的溶解度产物不一致。对于具有可变pH和磷酸盐浓度的溶液,IAP的高度可变性表明HPM的溶解-沉淀反应不能用平衡状态下的恒定溶解度产物来描述,这可能是由于该矿物在接近中性pH值下的溶解行为不一致所致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号