当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In(III)‐Catalyzed O‐Annulation of Cyclic Diazodicarbonyls with 2‐Naphthol, 6‐Quinolinol, β‐Tetralone, and 9‐Phenanthrol to Access Diverse Benzochromones
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-01-04 , DOI: 10.1002/bkcs.12207 Shizuka Mei Bautista Maezono 1 , Hari Datta Khanal 1 , Priyanka Chaudhary 1 , Ga Eul Park 1 , Yong Rok Lee 1
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-01-04 , DOI: 10.1002/bkcs.12207 Shizuka Mei Bautista Maezono 1 , Hari Datta Khanal 1 , Priyanka Chaudhary 1 , Ga Eul Park 1 , Yong Rok Lee 1
Affiliation
|
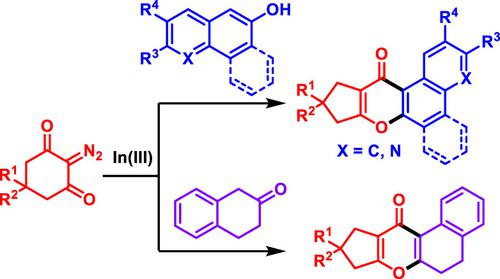
In(III)‐Catalyzed O‐annulation of cyclic diazodicarbonyl compounds with 2‐naphthols, 6‐quinolinol, β‐tetralone, and 9‐phenanthrol is developed. This novel synthetic strategy paves a facile and desired access to diverse and functionalized benzochromones. This imperative protocol involves domino carbene generation, ketene formation through Wolff‐rearrangement, nucleophilic addition, H‐migration, intramolecular cyclization, and elimination of water.
中文翻译:

在In(III)催化下的环重氮二羰基与2-萘酚,6-喹啉醇,β-四氢萘酮和9-菲咯啉的O型环化反应获得各种苯并色酮
在(III)催化下,环状重氮二羰基化合物与2-萘酚,6-喹啉醇,β-四氢萘酮和9-菲咯啉的O环化反应得以发展。这种新颖的合成策略为轻松获得各种功能化的苯并色酮提供了所需的途径。该命令性协议涉及多米诺卡宾的产生,通过Wolff重排形成烯酮,亲核加成,H迁移,分子内环化和除水。
更新日期:2021-01-04
中文翻译:

在In(III)催化下的环重氮二羰基与2-萘酚,6-喹啉醇,β-四氢萘酮和9-菲咯啉的O型环化反应获得各种苯并色酮
在(III)催化下,环状重氮二羰基化合物与2-萘酚,6-喹啉醇,β-四氢萘酮和9-菲咯啉的O环化反应得以发展。这种新颖的合成策略为轻松获得各种功能化的苯并色酮提供了所需的途径。该命令性协议涉及多米诺卡宾的产生,通过Wolff重排形成烯酮,亲核加成,H迁移,分子内环化和除水。


























 京公网安备 11010802027423号
京公网安备 11010802027423号