Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2021-01-05 , DOI: 10.1016/j.bioorg.2020.104611 Sarvesh Kumar Pandey 1 , Umesh Yadava 2 , Anjali Upadhyay 1 , M L Sharma 3
|
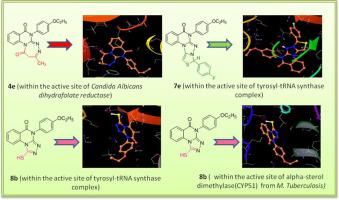
In the present study, a series of novel quinazolinone hybrids, viz. triazepino-quinazolinones 4, thiazolo-triazolo-quinazolinones 7 and triazolo-quinazolinones 8 have been synthesized from the key intermediate 3-(substituted phenyl)-2-hydrazinoquinazolin-4(3H)-ones 3. All the newly synthesized compounds were characterized by means of spectral (IR, 1H NMR, 13C NMR) and elemental analysis. The target compounds were biologically screened for their in vitro antimicrobial and antitubercular activities against pathogenic strain. The results of bioassay demonstrated that some of the compounds exhibited pronounced antimicrobial activity comparable to that of standard drugs tested under similar conditions. Compounds 4c, 4e, 7e and 8b showed relatively very good inhibitory activity against pathogenic bacteria with minimum inhibitory concentration (MIC) of 2.6 μg/mL, 5.2 μg/mL, while the rest of the compounds showed moderate activity. Compounds 4c and 8b were found to be nearly equipotent with ciprofloxacin against P. aeruginosa with MIC 5.2 μg/mL, while compound 8b was more potent against pathogenic bacteria S. aureus. It is very remarkable that four compounds, 4c, 4e, 7e and 8b showed pronounced antifungal activity against selected pathogenic fungi, A. niger, C. albicans with MIC 2.6 μg/mL and 5.2 μg/mL. The antitubercular activity of synthesized compounds reveal that compound 8b showed better activity than the other compounds with a MIC of 5.2 μg/mL against M. tuberculosis (H37Rv). Molecular docking studies of the compounds were performed to rationalize the inhibitory properties of these compounds and results showed that these compounds have good binding energy and better binding affinity within the active pocket, thus these compounds may be considered as potent inhibitors towards selective targets.
中文翻译:

新型喹唑啉酮类抗结核和抗菌药物的合成、生物学评价和分子对接研究
在本研究中,一系列新型喹唑啉酮杂化物,即。已经从关键中间体 3-(取代苯基)-2-肼基喹唑啉-4 (3H )-ones 3合成了三氮杂-喹唑啉酮4、噻唑并-三唑-喹唑啉酮7和三唑-喹唑啉酮8。所有新合成的化合物均通过光谱(IR、1 H NMR、13 C NMR)和元素分析进行表征。对目标化合物进行了体外生物学筛选对致病菌株的抗菌和抗结核活性。生物测定结果表明,某些化合物表现出与在类似条件下测试的标准药物相当的显着抗菌活性。化合物4c、4e、7e和8b对病原菌表现出较好的抑制活性,最低抑菌浓度(MIC)分别为2.6 μg/mL、5.2 μg/mL,其余化合物均表现出中等活性。发现化合物4c和8b与环丙沙星对铜绿假单胞菌几乎等效,MIC 为 5.2 μg/mL,而化合物8b对致病菌金黄色葡萄球菌更有效。非常值得注意的是,四种化合物4c、4e、7e和8b对选定的病原真菌、黑曲霉、白色念珠菌显示出显着的抗真菌活性,MIC 分别为 2.6 μg/mL 和 5.2 μg/mL。合成化合物的抗结核活性表明,化合物8b显示出比其他化合物更好的活性,MIC 为 5.2 μg/mL,对结核分枝杆菌(H 37RV)。进行了化合物的分子对接研究以合理化这些化合物的抑制特性,结果表明这些化合物在活性口袋内具有良好的结合能和更好的结合亲和力,因此这些化合物可被认为是针对选择性靶标的有效抑制剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号