Environmental Technology & Innovation ( IF 7.1 ) Pub Date : 2021-01-04 , DOI: 10.1016/j.eti.2020.101347 Nari Park , Hyangyoun Chang , Yeoju Jang , Hyunman Lim , Jinhong Jung , Weonjae Kim
|
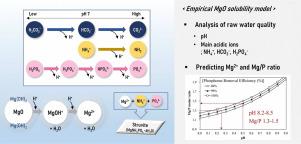
The struvite crystallization process can recover struvite crystals as valuable slow-release fertilizers from raw water, including high phosphate and ammonium ions. MgO can be used as a magnesium source and an alkaline chemical simultaneously; however, it is difficult to control the dissolved Mg concentration because of its insufficient and unpredictable solubility. The purpose of this study was to estimate MgO solubility and determine the optimal operating conditions of pH and Mg dosage during struvite crystallization. An empirical MgO solubility prediction model was devised to dose an adequate amount of MgO slurry in accordance with the properties of raw water. MgO solubility was determined by H ions donated by NH, HCO, and HPO as the pH increased in the solution. It was found that the molar ratio of donated H to dissolved Mg converged to 2:1. This model was verified by performing jar-tests using digested sludge filtrate. When adjusting to a pH of 8.0–9.3 using only MgO, the predicted Mg concentrations using the model were compared to the measured Mg concentrations, and they coincided well within the maximum error of 5.7%. This model was applied to determining the operating conditions of pH and Mg dosage. As a result, considering phosphate removal efficiency higher than 90%, it was feasible to simultaneously achieve an Mg/P molar ratio of 1.3–1.5 and a pH of 8.2–8.5 by only using MgO. Therefore, MgO is recommendable as an appropriate magnesium source and alkali supplier in the struvite process based on experimental jar-tests and the empirical MgO solubility model.
中文翻译:

预测足够的pH和Mg 使用经验性MgO溶解度模型的鸟粪石结晶剂量
鸟粪石结晶过程可以从原水中回收鸟粪石晶体,作为有价值的缓释肥料,包括高磷酸盐和铵离子。MgO可以同时用作镁源和碱性化学品。但是,很难控制溶解的镁由于其溶解度不足且无法预测,因此浓度较高。这项研究的目的是评估MgO的溶解度并确定pH和Mg的最佳操作条件鸟粪石结晶过程中的剂量。设计了一个经验性的MgO溶解度预测模型,以根据原水的特性添加适量的MgO浆料。MgO溶解度由H确定 NH捐赠的离子,HCO和HPO随着溶液中pH值的增加。发现捐赠的H的摩尔比 溶解镁收敛到2:1。通过使用消化的污泥滤液进行广口瓶测试,验证了该模型。仅使用MgO将pH调节至8.0–9.3时,预测的Mg 使用模型的浓度与测得的Mg进行比较浓度,并且它们恰好在5.7%的最大误差内。该模型用于确定pH和Mg的操作条件剂量。结果,考虑到磷酸盐去除效率高于90%,仅使用MgO同时达到1.3-1.5的Mg / P摩尔比和8.2-8.5的pH是可行的。因此,基于实验震击试验和经验性MgO溶解度模型,MgO被推荐为鸟粪石工艺中合适的镁源和碱供应商。


























 京公网安备 11010802027423号
京公网安备 11010802027423号