当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How Tryptophan Oxidation Arises by “Dark” Photoreactions from Chemiexcited Triplet Acetone
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-01-21 , DOI: 10.1111/php.13375 Ryan M O'Connor 1 , Alexander Greer 1, 2
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-01-21 , DOI: 10.1111/php.13375 Ryan M O'Connor 1 , Alexander Greer 1, 2
Affiliation
|
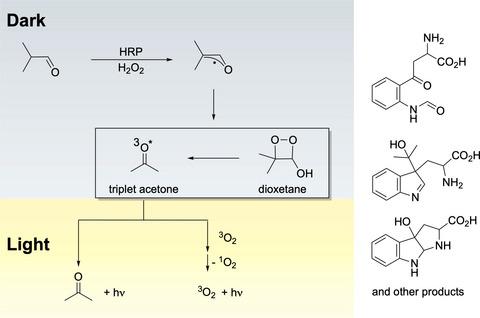
Dioxetane intermediates readily decompose to chemiluminescent triplet carbonyls, giving rise to what has been paradoxically called photochemistry in the dark. In this issue of Photochemistry and Photobiology, Bechara et al. report on mechanistic advances in such a reaction. With the use of horseradish peroxidase for isobutyraldehyde-derived triplet acetone, light emission from acetone and singlet oxygen can be quenched. The experiments reveal that the reaction depends on oxygen and the amino acid. The analysis reveals that free tryptophan is a target of this form of "carbonyl stress", with the efficient formation of mono-, bi- and tricyclic compounds (N-formylkynurenine, indoline, 1l2 -indole, and 3H-indoles).
中文翻译:

来自化学激发的三重丙酮的“暗”光反应如何引起色氨酸氧化
二氧杂环丁烷中间体很容易分解成化学发光的三线态羰基化合物,从而产生了在黑暗中被矛盾地称为光化学的现象。在本期光化学和光生物学中,Bechara 等人。报告这种反应的机制进展。通过使用辣根过氧化物酶制备异丁醛衍生的三线态丙酮,可以抑制丙酮和单线态氧的发光。实验表明,该反应依赖于氧气和氨基酸。分析表明,游离色氨酸是这种“羰基应激”形式的目标,可有效形成单环、双环和三环化合物(N-甲酰基犬尿氨酸、二氢吲哚、1l2-吲哚和 3H-吲哚)。
更新日期:2021-01-21
中文翻译:

来自化学激发的三重丙酮的“暗”光反应如何引起色氨酸氧化
二氧杂环丁烷中间体很容易分解成化学发光的三线态羰基化合物,从而产生了在黑暗中被矛盾地称为光化学的现象。在本期光化学和光生物学中,Bechara 等人。报告这种反应的机制进展。通过使用辣根过氧化物酶制备异丁醛衍生的三线态丙酮,可以抑制丙酮和单线态氧的发光。实验表明,该反应依赖于氧气和氨基酸。分析表明,游离色氨酸是这种“羰基应激”形式的目标,可有效形成单环、双环和三环化合物(N-甲酰基犬尿氨酸、二氢吲哚、1l2-吲哚和 3H-吲哚)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号