当前位置:
X-MOL 学术
›
Biol. Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
AtFH14 crosslinks actin filaments and microtubules in different manners
Biology of the Cell ( IF 2.7 ) Pub Date : 2021-02-01 , DOI: 10.1111/boc.202000147 Pingzhou Du 1 , Jiaojiao Wang 1 , Yunqiu He 1 , Sha Zhang 1 , Bailing Hu 1 , Xiuhua Xue 1 , Long Miao 2, 3 , Haiyun Ren 1
Biology of the Cell ( IF 2.7 ) Pub Date : 2021-02-01 , DOI: 10.1111/boc.202000147 Pingzhou Du 1 , Jiaojiao Wang 1 , Yunqiu He 1 , Sha Zhang 1 , Bailing Hu 1 , Xiuhua Xue 1 , Long Miao 2, 3 , Haiyun Ren 1
Affiliation
|
BACKGROUND INFORMATION
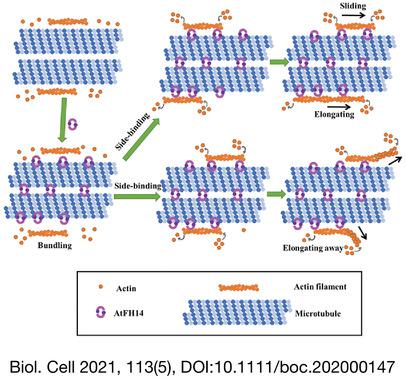
In many cellular processes including cell division, the synergistic dynamics of actin filaments and microtubules play vital roles. However, the regulatory mechanisms of these synergistic dynamics are not fully understood. Proteins such as formins are involved in actin filament-microtubule interactions and Arabidopsis thaliana formin 14 (AtFH14) may function as a crosslinker between actin filaments and microtubules in cell division, but the molecular mechanism underlying such crosslinking remains unclear. RESULTS
Without microtubules, formin homology (FH) 1/FH2 of AtFH14 nucleated actin polymerization from actin monomers and capped the barbed end of actin filaments. However, in the presence of microtubules, quantitative analysis showed that the binding affinity of AtFH14 FH1FH2 to microtubules was higher than that to actin filaments. Moreover, microtubule-bound AtFH14 FH1FH2 neither nucleated actin polymerization nor inhibited barbed end elongation. In contrast, tubulin did not affect AtFH14 FH1FH2 to nucleate actin polymerization and inhibit barbed end elongation. Nevertheless, microtubule-bound AtFH14 FH1FH2 bound actin filaments and the bound actin filaments slid and elongated along the microtubules or elongated away from the microtubules, which induced bundling or crosslinking of actin filaments and microtubules. Pharmacological analyses indicated that AtFH14 FH1FH2 promoted crosslinking of actin filaments and microtubules in vivo. Additionally, cosedimentation and fluorescent dye-labeling experiments of AtFH14 FH2-truncated proteins in vitro revealed the essential motifs of bundling actin filaments or microtubules, which were 63-92 aa and 42-62 aa in the AtFH14 FH2 N-terminal, respectively, and 42-62 aa was the essential motif to crosslink actin filaments and microtubules. CONCLUSIONS AND SIGNIFICANCE
Our results aid in explaining how AtFH14 functions as a crosslinker between actin filaments and microtubules to regulate their dynamics via different manners during cell division. They also facilitate further understanding of the molecular mechanisms of the interactions between actin filaments and microtubules. This article is protected by copyright. All rights reserved.
中文翻译:

AtFH14 以不同方式交联肌动蛋白丝和微管
背景信息在包括细胞分裂在内的许多细胞过程中,肌动蛋白丝和微管的协同动力学起着至关重要的作用。然而,这些协同动态的调节机制尚未完全了解。肌动蛋白丝-微管相互作用中涉及诸如formins之类的蛋白质,拟南芥formin 14(AtFH14)可能在细胞分裂中充当肌动蛋白丝和微管之间的交联剂,但这种交联的分子机制尚不清楚。结果在没有微管的情况下,AtFH14 的formin homology (FH) 1/FH2 使肌动蛋白单体的肌动蛋白聚合成核,并覆盖了肌动蛋白丝的带刺末端。然而,在微管的存在下,定量分析表明,AtFH14 FH1FH2对微管的结合亲和力高于对肌动蛋白丝的结合亲和力。此外,微管结合的 AtFH14 FH1FH2 既不使肌动蛋白聚合成核,也不抑制倒刺末端伸长。相比之下,微管蛋白不影响 AtFH14 FH1FH2 使肌动蛋白聚合成核并抑制倒刺末端伸长。然而,微管结合的 AtFH14 FH1FH2 结合肌动蛋白丝,结合的肌动蛋白丝沿着微管滑动并拉长或远离微管拉长,这导致肌动蛋白丝和微管的捆绑或交联。药理学分析表明,AtFH14 FH1FH2 促进体内肌动蛋白丝和微管的交联。此外,AtFH14 FH2 截短蛋白的体外共沉淀和荧光染料标记实验揭示了捆绑肌动蛋白丝或微管的基本基序,它们分别在 AtFH14 FH2 N 端的 63-92 aa 和 42-62 aa,以及 42- 62 aa 是交联肌动蛋白丝和微管的基本基序。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。AtFH14 FH2 N-末端分别为 63-92 aa 和 42-62 aa,42-62 aa 是交联肌动蛋白丝和微管的基本基序。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。AtFH14 FH2 N-末端分别为 63-92 aa 和 42-62 aa,42-62 aa 是交联肌动蛋白丝和微管的基本基序。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。
更新日期:2021-02-01
中文翻译:

AtFH14 以不同方式交联肌动蛋白丝和微管
背景信息在包括细胞分裂在内的许多细胞过程中,肌动蛋白丝和微管的协同动力学起着至关重要的作用。然而,这些协同动态的调节机制尚未完全了解。肌动蛋白丝-微管相互作用中涉及诸如formins之类的蛋白质,拟南芥formin 14(AtFH14)可能在细胞分裂中充当肌动蛋白丝和微管之间的交联剂,但这种交联的分子机制尚不清楚。结果在没有微管的情况下,AtFH14 的formin homology (FH) 1/FH2 使肌动蛋白单体的肌动蛋白聚合成核,并覆盖了肌动蛋白丝的带刺末端。然而,在微管的存在下,定量分析表明,AtFH14 FH1FH2对微管的结合亲和力高于对肌动蛋白丝的结合亲和力。此外,微管结合的 AtFH14 FH1FH2 既不使肌动蛋白聚合成核,也不抑制倒刺末端伸长。相比之下,微管蛋白不影响 AtFH14 FH1FH2 使肌动蛋白聚合成核并抑制倒刺末端伸长。然而,微管结合的 AtFH14 FH1FH2 结合肌动蛋白丝,结合的肌动蛋白丝沿着微管滑动并拉长或远离微管拉长,这导致肌动蛋白丝和微管的捆绑或交联。药理学分析表明,AtFH14 FH1FH2 促进体内肌动蛋白丝和微管的交联。此外,AtFH14 FH2 截短蛋白的体外共沉淀和荧光染料标记实验揭示了捆绑肌动蛋白丝或微管的基本基序,它们分别在 AtFH14 FH2 N 端的 63-92 aa 和 42-62 aa,以及 42- 62 aa 是交联肌动蛋白丝和微管的基本基序。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。AtFH14 FH2 N-末端分别为 63-92 aa 和 42-62 aa,42-62 aa 是交联肌动蛋白丝和微管的基本基序。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。AtFH14 FH2 N-末端分别为 63-92 aa 和 42-62 aa,42-62 aa 是交联肌动蛋白丝和微管的基本基序。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。结论和意义我们的结果有助于解释 AtFH14 如何作为肌动蛋白丝和微管之间的交联剂发挥作用,以在细胞分裂过程中通过不同方式调节它们的动态。它们还有助于进一步了解肌动蛋白丝和微管之间相互作用的分子机制。本文受版权保护。版权所有。


























 京公网安备 11010802027423号
京公网安备 11010802027423号