Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-01-02 , DOI: 10.1016/j.bmc.2020.115960 Hoda Abolhasani 1 , Afshin Zarghi 2 , Tahereh Komeili Movahhed 3 , Ahmad Abolhasani 4 , Bahram Daraei 5 , Siavoush Dastmalchi 6
|
Objective
A new family of 3′-(Mono, di or tri-substituted phenyl)-4′-(4-(methylsulfonyl) phenyl) spiroisoxazoline derivatives containing indanone spirobridge was designed, synthesized, and evaluated for their selective COX-2 inhibitory potency and cytotoxicity on different cell lines.
Methods
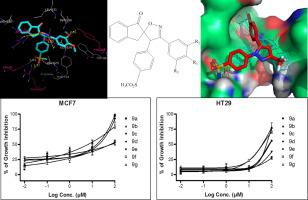
A synthetic reaction based on 1,3-dipolar cycloaddition mechanism was applied for the regiospecific formation of various spiroisoxazolines. The activity of the newly synthesized compounds was determined using in vitro cyclooxygenase inhibition assay. The toxicity of the compounds was evaluated by MTT assay. In addition, induction of apoptosis, and expression levels of Bax, Bcl-2 and caspase-3 mRNA in MCF-7 cells were evaluated following exposure to compound 9f. The docking calculations and molecular dynamics simulation were performed to study the most probable modes of interactions of compound 9f upon binding to COX-2 enzyme.
Results
The docking results showed that the synthesized compounds were able to form hydrogen bonds with COX-2 involving methyl sulfonyl, spiroisoxazoline, meta-methoxy and fluoro functional groups. Spiroisoxazoline derivatives containing methoxy group at the C-3′ phenyl ring meta position (9f and 9g) showed superior selectivity with higher potency of inhibiting COX-2 enzyme. Furthermore, compound 9f, which possesses 3,4-dimethoxyphenyl on C-3′ carbon atom of isoxazoline ring, exhibited the highest COX-2 inhibitory activity, and also displayed the most potent cytotoxicity on MCF-7 cells with an IC50 value of 0.03 ± 0.01 µM, comparable with that of doxorubicin (IC50 of 0.062 ± 0.012 µM). The results indicated that compound 9f could promote apoptosis. Also, compared to the control group, the mRNA expression of Bax and caspase-3 significantly increased, while that of Bcl-2 significantly decreased upon exposure to compound 9f which may propose the activation of mitochondrial-associated pathway as the mechanism of observed apoptosis.
Conclusion
In vitro biological evaluations accompanied with in silico studies revealed that indanone tricyclic spiroisoxazoline derivatives are good candidates for the development of new anti-inflammatory and anticancer (colorectal and breast) agents.
中文翻译:

具有选择性 COX-2 抑制作用的新型茚满酮螺异恶唑啉衍生物作为抗癌剂的设计、合成和生物学评价
客观的
设计、合成了含有茚满酮螺桥的 3'-(单、二或三取代苯基)-4'-(4-(甲基磺酰基)苯基)螺异恶唑啉衍生物的新家族,并评估了它们的选择性 COX-2 抑制效力和对不同细胞系的细胞毒性。
方法
基于 1,3-偶极环加成机制的合成反应被应用于各种螺异恶唑啉的区域特异性形成。使用体外环氧合酶抑制试验测定新合成化合物的活性。MTT法评价化合物的毒性。此外,在暴露于化合物9f 后评估了细胞凋亡的诱导以及 MCF-7 细胞中 Bax、Bcl-2 和 caspase-3 mRNA 的表达水平。进行对接计算和分子动力学模拟以研究化合物9f与 COX-2 酶结合后最可能的相互作用模式。
结果
对接结果表明合成的化合物能够与COX-2形成涉及甲基磺酰基、螺异恶唑啉、间甲氧基和氟官能团的氢键。在 C-3' 苯环间位(9f和9g)含有甲氧基的螺异恶唑啉衍生物显示出优异的选择性和更高的抑制 COX-2 酶的效力。此外,在异恶唑啉环的 C-3' 碳原子上具有 3,4-二甲氧基苯基的化合物9f表现出最高的 COX-2 抑制活性,并且对 MCF-7 细胞也表现出最强的细胞毒性,IC 50值为0.03 ± 0.01 µM,与阿霉素相当 (IC 500.062 ± 0.012 µM)。结果表明化合物9f可促进细胞凋亡。此外,与对照组相比,Bax 和 caspase-3 的 mRNA 表达显着增加,而 Bcl-2 的 mRNA 表达在暴露于化合物9f后显着降低,这可能表明线粒体相关途径的激活是观察到的细胞凋亡的机制。
结论
体外生物学评估和计算机模拟研究表明,茚满酮三环螺异恶唑啉衍生物是开发新型抗炎和抗癌(结肠直肠和乳腺癌)药物的良好候选物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号