当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aza‐Michael addition of 1,2‐diazoles to structurally diverse enones: Efficient methods toward β‐amino ketones
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-12-31 , DOI: 10.1002/jhet.4221 Saibabu Polina 1 , V. P. Rama Kishore Putta 1 , Raghuram Gujjarappa 2 , Prasad Pralhad Pujar 1 , Chandi C. Malakar 2
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-12-31 , DOI: 10.1002/jhet.4221 Saibabu Polina 1 , V. P. Rama Kishore Putta 1 , Raghuram Gujjarappa 2 , Prasad Pralhad Pujar 1 , Chandi C. Malakar 2
Affiliation
|
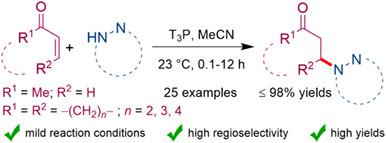
An efficient and mild protocol was realized using 1,2‐diazoles and related heterocycles with cyclic and acyclic enones in presence of T3P (2,4,6‐tripropyl‐1,3,5,2,4,6‐trioxatriphosphorinane‐2,4,6‐trioxide) toward the regioselective formation of N‐cycloalkyl heterocycles at room temperature. The developed reaction conditions showcased good selectivity over a wide range of 1,2‐diazoles and enones by delivering N‐cycloalkyl heterocycles in excellent yields.
中文翻译:

在结构多样的烯酮中添加1,2-二唑的Aza-Michael:对β-氨基酮的有效方法
在存在T3P(2,4,6-三丙基-1,3,5,2,4,6-三氧三磷酸正膦2, 4,6-三氧化物)在室温下朝向N-环烷基杂环的区域选择性形成。发达的反应条件通过以极高的产率提供N-环烷基杂环,在1,2-二唑和烯酮中显示出良好的选择性。
更新日期:2020-12-31
中文翻译:

在结构多样的烯酮中添加1,2-二唑的Aza-Michael:对β-氨基酮的有效方法
在存在T3P(2,4,6-三丙基-1,3,5,2,4,6-三氧三磷酸正膦2, 4,6-三氧化物)在室温下朝向N-环烷基杂环的区域选择性形成。发达的反应条件通过以极高的产率提供N-环烷基杂环,在1,2-二唑和烯酮中显示出良好的选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号