当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Orally Efficacious Allosteric Inhibitors of TNFα via Fragment-Based Drug Design
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-12-30 , DOI: 10.1021/acs.jmedchem.0c01280 Justin D. Dietrich 1 , Kenton L. Longenecker 1 , Noel S. Wilson 1 , Christian Goess 2 , Sanjay C. Panchal 1 , Steven L. Swann 1 , Andrew M. Petros 1 , Adrian D. Hobson 2 , David Ihle 2 , Danying Song 1 , Paul Richardson 1 , Kenneth M. Comess 1 , Philip B. Cox 1 , Amanda Dombrowski 1 , Kathy Sarris 1 , Diana L. Donnelly-Roberts 1 , David B. Duignan 2 , Arthur Gomtsyan 1 , Paul Jung 1 , A. Chris Krueger 1 , Suzanne Mathieu 2 , Andrea McClure 1 , Vincent S. Stoll 1 , Jill Wetter 1 , John A. Mankovich 2 , Philip J. Hajduk 1 , Anil Vasudevan 1 , Robert H. Stoffel 2 , Chaohong Sun 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-12-30 , DOI: 10.1021/acs.jmedchem.0c01280 Justin D. Dietrich 1 , Kenton L. Longenecker 1 , Noel S. Wilson 1 , Christian Goess 2 , Sanjay C. Panchal 1 , Steven L. Swann 1 , Andrew M. Petros 1 , Adrian D. Hobson 2 , David Ihle 2 , Danying Song 1 , Paul Richardson 1 , Kenneth M. Comess 1 , Philip B. Cox 1 , Amanda Dombrowski 1 , Kathy Sarris 1 , Diana L. Donnelly-Roberts 1 , David B. Duignan 2 , Arthur Gomtsyan 1 , Paul Jung 1 , A. Chris Krueger 1 , Suzanne Mathieu 2 , Andrea McClure 1 , Vincent S. Stoll 1 , Jill Wetter 1 , John A. Mankovich 2 , Philip J. Hajduk 1 , Anil Vasudevan 1 , Robert H. Stoffel 2 , Chaohong Sun 1
Affiliation
|
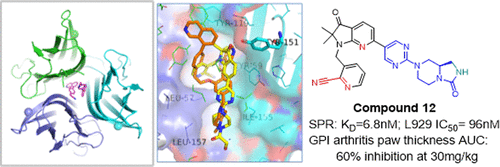
Tumor necrosis factor α (TNFα) is a soluble cytokine that is directly involved in systemic inflammation through the regulation of the intracellular NF-κB and MAPK signaling pathways. The development of biologic drugs that inhibit TNFα has led to improved clinical outcomes for patients with rheumatoid arthritis and other chronic autoimmune diseases; however, TNFα has proven to be difficult to drug with small molecules. Herein, we present a two-phase, fragment-based drug discovery (FBDD) effort in which we first identified isoquinoline fragments that disrupt TNFα ligand–receptor binding through an allosteric desymmetrization mechanism as observed in high-resolution crystal structures. The second phase of discovery focused on the de novo design and optimization of fragments with improved binding efficiency and drug-like properties. The 3-indolinone-based lead presented here displays oral, in vivo efficacy in a mouse glucose-6-phosphate isomerase (GPI)-induced paw swelling model comparable to that seen with a TNFα antibody.
中文翻译:

通过基于片段的药物设计开发口服有效的TNFα变构抑制剂
肿瘤坏死因子α(TNFα)是一种可溶性细胞因子,通过调节细胞内NF-κB和MAPK信号通路直接参与全身性炎症。抑制TNFα的生物药物的开发已使类风湿关节炎和其他慢性自身免疫性疾病的患者的临床预后得到改善。然而,事实证明,TNFα难以用小分子进行药物治疗。本文中,我们提出了一个基于片段的两阶段药物发现(FBDD)研究,在该研究中,我们首先鉴定了异喹啉片段,这些片段通过高分辨率晶体结构中观察到的变构去对称化机制破坏了TNFα配体-受体的结合。发现的第二阶段集中在从头开始设计和优化具有提高的结合效率和类药物特性的片段。此处介绍的基于3-indolinone的导线在小鼠葡萄糖-6-磷酸异构酶(GPI)诱导的爪肿胀模型中显示出与TNFα抗体相当的口服,体内功效。
更新日期:2021-01-14
中文翻译:

通过基于片段的药物设计开发口服有效的TNFα变构抑制剂
肿瘤坏死因子α(TNFα)是一种可溶性细胞因子,通过调节细胞内NF-κB和MAPK信号通路直接参与全身性炎症。抑制TNFα的生物药物的开发已使类风湿关节炎和其他慢性自身免疫性疾病的患者的临床预后得到改善。然而,事实证明,TNFα难以用小分子进行药物治疗。本文中,我们提出了一个基于片段的两阶段药物发现(FBDD)研究,在该研究中,我们首先鉴定了异喹啉片段,这些片段通过高分辨率晶体结构中观察到的变构去对称化机制破坏了TNFα配体-受体的结合。发现的第二阶段集中在从头开始设计和优化具有提高的结合效率和类药物特性的片段。此处介绍的基于3-indolinone的导线在小鼠葡萄糖-6-磷酸异构酶(GPI)诱导的爪肿胀模型中显示出与TNFα抗体相当的口服,体内功效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号