Water Research ( IF 12.8 ) Pub Date : 2020-12-30 , DOI: 10.1016/j.watres.2020.116797 Sijia Liang , Shuxia Xu , Chao Wang , Jingyi Ling , Zeyu Xian , Hao Wu , Haoting Tian , Shaoda Zhou , Cheng Gu
|
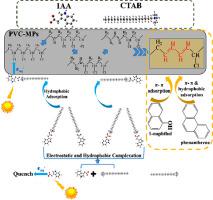
In this study, a new photo-irradiated reductive dechlorination pathway and the underlying transformation mechanism are described for poly(vinyl chloride) microplastics (PVC-MPs). PVC-MPs underwent photo-reductive dechlorination process with the release of chloride ions. This reaction could be facilitated in the presence of indole-3-acetic acid (IAA) and hexadecyltrimethylammonium bromide (CTAB) under neutral pH and simulated sunlight irradiation conditions. Electrostatic interaction between IAA and CTAB produced neutral IAA/CTAB complex, which might account for the enhanced adsorption of IAA on PVC powders. Upon photo-irradiation, the adsorbed IAA was excited to generate hydrated electrons (eaq−), which could pass through a shorter distance to PVC-MP surface than that derived from homogeneous IAA molecules in aqueous solution. Transient spectra of laser flash photolysis provided direct evidence for the generation of eaq−, which supported the proposed dechlorination mechanism. Based on the results of attenuated total reflectance/Fourier transform infrared (ATR/FTIR) and Raman spectra, C-Cl bond cleavage and polyene formation were involved in the structural transformation of PVC-MPs. Due to the hydrophobic effects and π-π interactions between aromatic rings and polyene structures in PVC-MP surface, the PVC-MP powders irradiated in the presence of IAA/CTAB showed an enhanced sorption for both hydrophobic and hydrophilic aromatic chemicals.
中文翻译:

由常见的阳离子表面活性剂辅助的吲哚-3-乙酸衍生的水合电子增强了聚氯乙烯微塑料的改性
在这项研究中,描述了一种新的光辐照还原性脱氯途径及其潜在的转化机理,用于聚氯乙烯微塑料(PVC-MPs)。PVC-MP经过光还原脱氯过程,释放出氯离子。在中性pH和模拟阳光照射条件下,在吲哚-3-乙酸(IAA)和十六烷基三甲基溴化铵(CTAB)的存在下可以促进该反应。IAA和CTAB之间的静电相互作用产生了中性的IAA / CTAB复合物,这可能解释了IAA在PVC粉体上的吸附增强。在光照射时,该吸附IAA被激发,以产生水合的电子(e水溶液-),它通过PVC-MP表面的距离比水溶液中均质IAA分子的距离短。激光闪光光解瞬态谱电子的产生提供了直接的证据含水- ,它支持拟议的脱氯机制。基于全反射衰减/傅立叶变换红外光谱(ATR / FTIR)和拉曼光谱的结果,C-Cl键断裂和多烯形成参与了PVC-MP的结构转变。由于PVC-MP表面芳香环与多烯结构之间的疏水作用和π-π相互作用,在IAA / CTAB存在下辐照的PVC-MP粉末对疏水和亲水芳香族化学物质的吸附均增强。



























 京公网安备 11010802027423号
京公网安备 11010802027423号