Journal of Structural Biology ( IF 3 ) Pub Date : 2020-12-30 , DOI: 10.1016/j.jsb.2020.107692 Bankala Krishnarjuna 1 , Punnepalli Sunanda 1 , Jessica Villegas-Moreno 2 , Agota Csoti 3 , Rodrigo A V Morales 1 , Dorothy C C Wai 1 , Gyorgy Panyi 3 , Peter Prentis 4 , Raymond S Norton 5
|
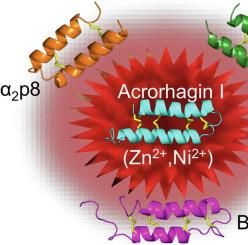
Acrorhagin I (U-AITX-Aeq5a) is a disulfide–rich peptide identified in the aggressive organs (acrorhagi) of the sea anemone Actinia equina. Previous studies (Toxicon 2005, 46:768–74) found that the peptide is toxic in crabs, although the structural and functional properties of acrorhagin I have not been reported. In this work, an Escherichia coli (BL21 strain) expression system was established for the preparation of 13C,15N–labelled acrorhagin I, and the solution structure was determined using NMR spectroscopy. Structurally, acrorhagin I is similar to B–IV toxin from the marine worm Cerebratulus lacteus (PDB id 1VIB), with a well–defined helical hairpin structure stabilised by four intramolecular disulfide bonds. The recombinant peptide was tested in patch–clamp electrophysiology assays against voltage-gated potassium and sodium channels, and in bacterial and fungal growth inhibitory assays and haemolytic assays. Acrorhagin I was not active against any of the ion channels tested and showed no activity in functional assays, indicating that this peptide may possess a different biological function. Metal ion interaction studies using NMR spectroscopy showed that acrorhagin I bound zinc and nickel, suggesting that its function might be modulated by metal ions or that it may be involved in regulating metal ion levels and their transport. The similarity between the structure of acrorhagin I and that of B-IV toxin from a marine worm suggests that this fold may prove to be a recurring motif in disulfide-rich peptides from marine organisms.
中文翻译:

acrorhagin I 中二硫化物稳定的螺旋发夹折叠:肽毒素中新兴的结构基序
Acrorhagin I (U-AITX-Aeq5a) 是一种富含二硫键的肽,存在于海葵Actinia equina的侵袭性器官 (acrorhagi) 中。先前的研究 ( Toxicon 2005, 46:768–74) 发现该肽在螃蟹中是有毒的,尽管尚未报道 acrorhagin I 的结构和功能特性。在这项工作中,建立了大肠杆菌(BL21 菌株)表达系统,用于制备13 C、15 N 标记的 acrorhagin I,并使用核磁共振光谱确定溶液结构。在结构上,acorhagin I 类似于来自海洋蠕虫Cerebratulus lacteus (PDB id 1VIB)的 B-IV 毒素,具有由四个分子内二硫键稳定的明确的螺旋发夹结构。重组肽在针对电压门控钾和钠通道的膜片钳电生理学分析、细菌和真菌生长抑制分析和溶血分析中进行了测试。Acrorhagin I 对所测试的任何离子通道均无活性,并且在功能测定中没有显示活性,表明该肽可能具有不同的生物学功能。使用 NMR 光谱进行的金属离子相互作用研究表明 acrorhagin I 与锌和镍结合,表明其功能可能受到金属离子的调节,或者它可能参与调节金属离子水平及其运输。



























 京公网安备 11010802027423号
京公网安备 11010802027423号