Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-12-30 , DOI: 10.1016/j.molliq.2020.115199 Reem M. Alghanmi , Maram T. Basha , Saied M. Soliman , Razan K. Alsaeedi
|
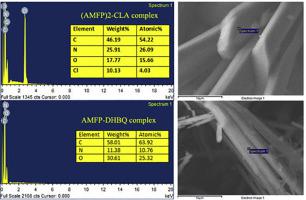
The charge transfer (CT) complexes formed between the potassium-channel-blocking drug amifampridine (AMFP) and the π-acceptors; chloranilic acid (CLA) and 2,5-dihydroxy-p-benzoquinone (DHBQ) in both MeOH solutions, and the solid-state were synthesized and fully characterized. The stoichiometry of the complexes formed in MeOH is 1:1, while those of the solid complexes are 2:1 and 1:1 (donor: acceptor) for CLA and DHBQ, respectively. The CT complexes were studied in terms of their formation constants (KCT), molar extinction coefficients (εCT), and spectrophysical and thermodynamic properties, and the results demonstrated the high stability of the complexes in MeOH. The limits of detection and quantification for the complexes by UV–Vis spectrophotometry were also determined. The surface morphologies, particle sizes, and elemental compositions of the complexes were determined, utilizing X-ray powder diffraction, scanning electron microscope, and energy-dispersive X-ray techniques. The molecular structures, and the electronic properties of the complexes were investigated using B3LYP and WB97XD DFT calculations employing 6–31++G(d,p) basis sets, revealing that the magnitude of the CT is 0.357 e in AMFP-CLA and 0.317 e in AMFP-DHBQ, indicating that CLA is a better electron acceptor than DHBQ. The UV–Vis spectral bands observed for AMFP-CLA and AMFP-DHBQ experimentally in MeOH at 488 and 535 nm, respectively, while these bands were calculated at 493.0 at 535.6 nm, respectively, in the same solvent (MeOH). Both bands were found to be due to HOMO→LUMO excitation, where that in the AMFP-CLA complex is an internal electronic transition and that in AMFP-DHBQ complex is a charge transfer based transition from AMFP to DHBQ. Natural bond orbital calculations indicated that the CT processes LP(2)O11→LP*(1)H12 (127.62 kcal mol−1) and LP(1)N20→LP*(1)H12 (317.46 kcal mol−1) in AMFP-DHBQ, and LP(2)O9→LP*(1)H10 (94.84 kcal mol−1) and LP(1)N23→ LP*(1)H10 (363.42 kcal mol−1) in AMFP-CLA provide extra stability to the complexes.
中文翻译:

氨苯丙啶与π受体的新型电荷转移配合物的合成,光谱,纳米结构,表面形貌和密度泛函理论研究
钾通道阻断药物氨磷if啶(AMFP)与π受体之间形成电荷转移(CT)配合物;在两种MeOH溶液中合成氯苯甲酸(CLA)和2,5-二羟基对苯醌(DHBQ),并对其固态进行了充分表征。在MeOH中形成的配合物的化学计量比为1:1,而对于CLA和DHBQ,固体配合物的化学计量比分别为2:1和1:1(供体:受体)。的CT络合物在它们的形成常数(方面进行了研究ķ CT),摩尔消光系数(ε CT),光谱物理和热力学性质,结果证明了该配合物在MeOH中的高稳定性。还确定了通过紫外可见分光光度法检测和定量复合物的极限。利用X射线粉末衍射,扫描电子显微镜和能量色散X射线技术确定了配合物的表面形貌,粒径和元素组成。使用B3LYP和WB97XD DFT计算(使用6-31 ++ G(d,p)基集)研究了配合物的分子结构和电子性质,结果表明,在AMFP-CLA中,CT的大小为0.357 e,在0.317 e中AMFP-DHBQ中的e,表明CLA比DHBQ是更好的电子受体。在甲醇中分别在488和535 nm处分别通过实验观察到AMFP-CLA和AMFP-DHBQ的UV-Vis光谱带,而在相同溶剂(MeOH)中,分别在535.6 nm下在493.0处计算出了这些光谱带。发现这两个带都是由于HOMO→LUMO激发引起的,其中AMFP-CLA络合物中的是内部电子跃迁,而AMFP-DHBQ络合物中的是基于电荷转移的从AMFP到DHBQ的跃迁。自然键轨道计算表明CT在AMFP中处理LP(2)O11→LP *(1)H12(127.62 kcal mol -1)和LP(1)N20→LP *(1)H12(317.46 kcal mol -1)-DHBQ,并且AMFP-CLA中的LP(2)O9→LP *(1)H10(94.84 kcal mol -1)和LP(1)N23→LP *(1)H10(363.42 kcal mol -1)为AMFP-CLA提供了额外的稳定性复合体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号