当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorptive Removal of Unsaturated Fatty Acids Using Ion Exchange Resins
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-29 , DOI: 10.1021/acs.jced.0c00700 Manisha A. Khedkar 1 , Satchidanand R. Satpute 2 , Sandip B. Bankar 3 , Prakash V. Chavan 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-29 , DOI: 10.1021/acs.jced.0c00700 Manisha A. Khedkar 1 , Satchidanand R. Satpute 2 , Sandip B. Bankar 3 , Prakash V. Chavan 1
Affiliation
|
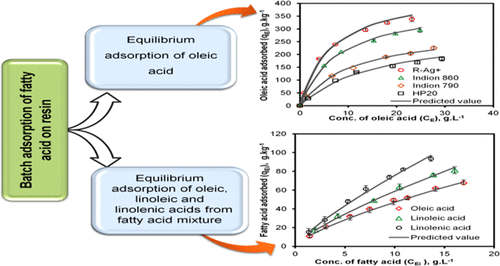
The main aim of the present investigation was to elucidate the efficacy of silver ion chromatography for selective separation of unsaturated (oleic, linoleic, and linolenic) fatty acids on a preparative scale. Accordingly, the present work was predominantly divided into two parts. In the first part, adsorption of oleic acid was carried out using commercially available ion exchange resins and silver-ion-loaded resins (R-Ag+) prepared in the laboratory from nonpolar and polar solvents in a batch mode. The maximum adsorption of oleic acid was found on R-Ag+ (454.55 g·kg–1) compared with other commercially available ion exchange resins from heptane at ambient temperature (303 K). The effect of temperature on the adsorption of oleic acid on R-Ag+ from heptane was investigated at 303, 313, and 323 K. The adsorption of oleic acid was favored at 303 K and decreased with a further increase in temperature. Experimental batch equilibrium data were modeled using the Langmuir and Freundlich isotherms. Further, thermodynamic parameters viz., ΔGads0, ΔHads0, and ΔSads0, were estimated. The negative values of ΔGads0 and ΔHads0 show that the adsorption of oleic acid on R-Ag+ was spontaneous and exothermic in nature. Based on the results obtained in the first part, the R-Ag+ resin was subjected to adsorption of fatty acids from industrial fatty acids mixture using heptane as a solvent at 303 K. A multicomponent Freundlich isotherm was used to model experimental batch equilibrium data. Linolenic acid and linoleic acid were preferentially adsorbed over oleic acid with selectivities of 1.40 and 1.16, respectively, from industrial fatty acids mixture.
中文翻译:

使用离子交换树脂吸附去除不饱和脂肪酸
本研究的主要目的是阐明银离子色谱在制备规模上选择性分离不饱和脂肪酸(油酸,亚油酸和亚麻酸)的功效。因此,目前的工作主要分为两个部分。在第一部分中,使用市售的离子交换树脂和载有银离子的树脂(R-Ag +)以分批方式在实验室中从非极性和极性溶剂中制备来吸附油酸。在室温(303 K)下,与庚烷中的其他市售离子交换树脂相比,在R-Ag +(454.55 g·kg –1)上发现了最大的油酸吸附。温度对R-Ag +上油酸吸附的影响在303、313和323 K下研究了庚烷中的油酸。在303 K时,油酸的吸附较为有利,并随着温度的进一步升高而降低。实验批量平衡数据使用Langmuir和Freundlich等温线建模。此外,热力学参数即,ΔG广告0,ΔH广告0,和ΔS广告0,估计。ΔG的负值广告0和ΔH广告0表明,在R-的Ag油酸的吸附+是自发和本质上是放热。根据第一部分获得的结果,R-Ag +使用庚烷作为溶剂,在303 K下对树脂从工业脂肪酸混合物中进行脂肪酸吸附。多组分Freundlich等温线用于模拟实验批平衡数据。从工业脂肪酸混合物中分别以1.40和1.16的选择性优先吸附亚油酸和亚油酸,而不是油酸。
更新日期:2021-01-14
中文翻译:

使用离子交换树脂吸附去除不饱和脂肪酸
本研究的主要目的是阐明银离子色谱在制备规模上选择性分离不饱和脂肪酸(油酸,亚油酸和亚麻酸)的功效。因此,目前的工作主要分为两个部分。在第一部分中,使用市售的离子交换树脂和载有银离子的树脂(R-Ag +)以分批方式在实验室中从非极性和极性溶剂中制备来吸附油酸。在室温(303 K)下,与庚烷中的其他市售离子交换树脂相比,在R-Ag +(454.55 g·kg –1)上发现了最大的油酸吸附。温度对R-Ag +上油酸吸附的影响在303、313和323 K下研究了庚烷中的油酸。在303 K时,油酸的吸附较为有利,并随着温度的进一步升高而降低。实验批量平衡数据使用Langmuir和Freundlich等温线建模。此外,热力学参数即,ΔG广告0,ΔH广告0,和ΔS广告0,估计。ΔG的负值广告0和ΔH广告0表明,在R-的Ag油酸的吸附+是自发和本质上是放热。根据第一部分获得的结果,R-Ag +使用庚烷作为溶剂,在303 K下对树脂从工业脂肪酸混合物中进行脂肪酸吸附。多组分Freundlich等温线用于模拟实验批平衡数据。从工业脂肪酸混合物中分别以1.40和1.16的选择性优先吸附亚油酸和亚油酸,而不是油酸。


























 京公网安备 11010802027423号
京公网安备 11010802027423号