当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substituted 9-Diethylaminobenzo[a]phenoxazin-5-ones (Nile Red Analogues): Synthesis and Photophysical Properties
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-12-28 , DOI: 10.1021/acs.joc.0c02346 Mick Hornum , Mads W. Mulberg , Maria Szomek , Peter Reinholdt , Jonathan R. Brewer , Daniel Wüstner , Jacob Kongsted , Poul Nielsen
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-12-28 , DOI: 10.1021/acs.joc.0c02346 Mick Hornum , Mads W. Mulberg , Maria Szomek , Peter Reinholdt , Jonathan R. Brewer , Daniel Wüstner , Jacob Kongsted , Poul Nielsen
|
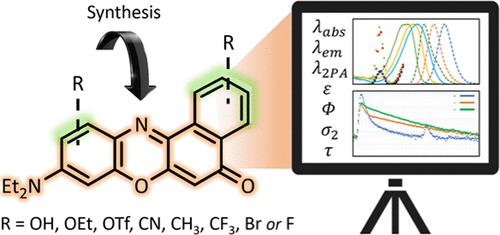
Nile Red is a benzo[a]phenoxazone dye containing a diethylamino substituent at the 9-position. In recent years, it has become a popular histological stain for cellular membranes and lipid droplets due to its unrivaled fluorescent properties in lipophilic environments. This makes it an attractive lead for chemical decoration to tweak its attributes and optimize it for more specialized microscopy techniques, e.g., fluorescence lifetime imaging or two-photon excited fluorescence microscopy, to which Nile Red has never been optimized. Herein, we present synthesis approaches to a series of monosubstituted Nile Red derivatives (9-diethylbenzo[a]phenoxazin-5-ones) starting from 1-naphthols or 1,3-naphthalenediols. The solvatochromic responsiveness of these fluorophores is reported with focus on how the substituents affect the absorption and emission spectra, luminosity, fluorescence lifetimes, and two-photon absorptivity. Several of the analogues emerge as strong candidates for reporting the polarity of their local environment. Specifically, the one- and two-photon excited fluorescence of Nile Red turns out to be very responsive to substitution, and the spectroscopic features can be finely tuned by judiciously introducing substituents of distinct electronic character at specific positions. This new toolkit of 9-diethylbenzo[a]phenoxazine-5-ones constitutes a step toward the next generation of optical molecular probes for advancing the understanding of lipid structures and cellular processes.
中文翻译:

取代的9-二乙基氨基苯并[ a ]苯恶嗪-5-酮(尼罗红类似物):合成和光物理性质
尼罗红是在9位含有二乙氨基取代基的苯并[ ]苯恶a酮染料。近年来,由于其在亲脂性环境中无与伦比的荧光特性,它已成为细胞膜和脂滴的流行组织学染色剂。这使其成为化学装饰的诱人线索,可以调整其属性并针对更专门的显微镜技术对其进行优化,例如荧光寿命成像或双光子激发荧光显微镜,而尼罗红从未对其进行优化。在这里,我们提出了一系列单取代的尼罗红衍生物(9-二乙基苯并[ a][phenoxazin-5-ones)起始于1-萘酚或1,3-萘二醇。报道了这些荧光团的溶剂变色响应性,重点是取代基如何影响吸收和发射光谱,发光度,荧光寿命和双光子吸收率。一些类似物成为报告其当地环境极性的强有力候选者。具体来说,尼罗红的单光子和双光子激发荧光证明对取代反应非常敏感,可以通过在特定位置明智地引入具有不同电子特性的取代基来精细调整光谱特征。这个新的9-二乙基苯并[ a]工具包] phenoxazine-5-ones迈向了下一代光学分子探针,以促进对脂质结构和细胞过程的理解。
更新日期:2021-01-16
中文翻译:

取代的9-二乙基氨基苯并[ a ]苯恶嗪-5-酮(尼罗红类似物):合成和光物理性质
尼罗红是在9位含有二乙氨基取代基的苯并[ ]苯恶a酮染料。近年来,由于其在亲脂性环境中无与伦比的荧光特性,它已成为细胞膜和脂滴的流行组织学染色剂。这使其成为化学装饰的诱人线索,可以调整其属性并针对更专门的显微镜技术对其进行优化,例如荧光寿命成像或双光子激发荧光显微镜,而尼罗红从未对其进行优化。在这里,我们提出了一系列单取代的尼罗红衍生物(9-二乙基苯并[ a][phenoxazin-5-ones)起始于1-萘酚或1,3-萘二醇。报道了这些荧光团的溶剂变色响应性,重点是取代基如何影响吸收和发射光谱,发光度,荧光寿命和双光子吸收率。一些类似物成为报告其当地环境极性的强有力候选者。具体来说,尼罗红的单光子和双光子激发荧光证明对取代反应非常敏感,可以通过在特定位置明智地引入具有不同电子特性的取代基来精细调整光谱特征。这个新的9-二乙基苯并[ a]工具包] phenoxazine-5-ones迈向了下一代光学分子探针,以促进对脂质结构和细胞过程的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号