Redox Biology ( IF 11.4 ) Pub Date : 2020-12-28 , DOI: 10.1016/j.redox.2020.101851 Verónica Miguel 1 , Ricardo Ramos 2 , Laura García-Bermejo 3 , Diego Rodríguez-Puyol 4 , Santiago Lamas 1
|
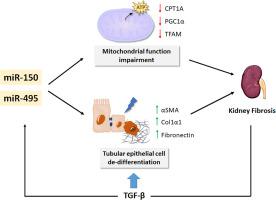
Excessive accumulation of extracellular matrix (ECM) is the hallmark of fibrotic diseases. In the kidney, it is the final common pathway of prevalent diseases, leading to chronic renal failure. While cytokines such as TGF-β play a fundamental role in myofibroblast transformation, recent work has shown that mitochondrial dysfunction and defective fatty acid oxidation (FAO), which compromise the main source of energy for renal tubular epithelial cells, have been proposed to be fundamental contributors to the development and progression of kidney fibrosis. MicroRNAs (miRNAs), which regulate gene expression post-transcriptionally, have been reported to control renal fibrogenesis. To identify miRNAs involved in the metabolic derangement of renal fibrosis, we performed a miRNA array screen in the mouse model of unilateral ureteral obstruction (UUO). MiR-150-5p and miR-495-3p were selected for their link to human pathology, their role in mitochondrial metabolism and their targeting of the fatty acid shuttling enzyme CPT1A. We found a 2- and 4-fold upregulation of miR-150-5p and miR-495-5p, respectively, in both the UUO and the folic acid induced nephropathy (FAN) models, while TGF-β1 upregulated their expressions in the human renal tubular epithelial cell line HKC-8. These miRNAs synergized with TGF-β regarding its pro-fibrotic effect by enhancing the fibrosis-associated markers Acta2, Col1α1 and Fn1. Bioenergetics studies showed a reduction of FAO-associated oxygen consumption rate (OCR) in HKC-8 cells in the presence of both miRNAs. Consistently, expression levels of their mitochondrial-related target genes CPT1A, PGC1α and the mitochondrial transcription factor A (TFAM), were reduced by half in renal epithelial cells exposed to these miRNAs. By contrast, we did not detect changes in mitochondrial mass and transmembrane potential (ΔѰm) or mitochondrial superoxide radical anion production. Our data support that miR-150 and miR-495 may contribute to renal fibrogenesis by aggravating the metabolic failure critically involved in tubular epithelial cells, ultimately leading to fibrosis.
中文翻译:

肾纤维化的程序由调控氧化代谢的微小RNA控制
细胞外基质(ECM)的过度积累是纤维化疾病的标志。在肾脏中,它是普遍疾病的最终常见途径,可导致慢性肾功能衰竭。尽管TGF-β等细胞因子在成肌纤维细胞转化中起着基本作用,但最近的研究表明,线粒体功能障碍和脂肪酸氧化缺陷(FAO)损害了肾小管上皮细胞的主要能量来源,已被认为是根本性的有助于肾纤维化的发展和进展。已经报道了转录后调节基因表达的MicroRNA(miRNA)控制肾纤维化。为了鉴定参与肾纤维化代谢紊乱的miRNA,我们在单侧输尿管阻塞(UUO)小鼠模型中进行了miRNA阵列筛选。选择MiR-150-5p和miR-495-3p是因为它们与人类病理学有关,在线粒体代谢中的作用以及对脂肪酸穿梭酶CPT1A的靶向作用。我们在UUO和叶酸诱导的肾病(FAN)模型中分别发现miR-150-5p和miR-495-5p的2倍和4倍上调,而TGF-β1上调了它们在人类中的表达肾小管上皮细胞系HKC-8。这些miRNA通过增强与纤维化相关的标志物Acta2,Col1α1和Fn1而与TGF-β协同发挥促纤维化作用。生物能学研究表明,在两种miRNA均存在的情况下,HKC-8细胞中与粮农组织相关的耗氧率(OCR)降低。一致地,其线粒体相关靶基因CPT1A,PGC1α和线粒体转录因子A(TFAM)的表达水平,在暴露于这些miRNA的肾上皮细胞中,这些蛋白的表达降低了一半。相比之下,我们没有检测到线粒体质量和跨膜电位(ΔѰm)或线粒体超氧自由基自由基产生的变化。我们的数据支持miR-150和miR-495可能会加剧肾小管上皮细胞严重参与的代谢衰竭,最终导致纤维化,从而有助于肾脏纤维化。

























 京公网安备 11010802027423号
京公网安备 11010802027423号