Cell ( IF 64.5 ) Pub Date : 2020-12-28 , DOI: 10.1016/j.cell.2020.11.049 Guanghui Yang 1 , Rui Zhou 2 , Xuefei Guo 2 , Chuangye Yan 2 , Jianlin Lei 3 , Yigong Shi 4
|
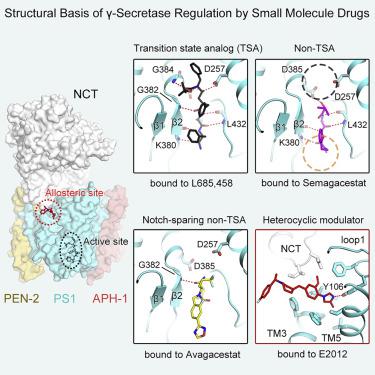
Development of γ-secretase inhibitors (GSIs) and modulators (GSMs) represents an attractive therapeutic opportunity for Alzheimer’s disease (AD) and cancers. However, how these GSIs and GSMs target γ-secretase has remained largely unknown. Here, we report the cryoelectron microscopy (cryo-EM) structures of human γ-secretase bound individually to two GSI clinical candidates, Semagacestat and Avagacestat, a transition state analog GSI L685,458, and a classic GSM E2012, at overall resolutions of 2.6–3.1 Å. Remarkably, each of the GSIs occupies the same general location on presenilin 1 (PS1) that accommodates the β strand from amyloid precursor protein or Notch, interfering with substrate recruitment. L685,458 directly coordinates the two catalytic aspartate residues of PS1. E2012 binds to an allosteric site of γ-secretase on the extracellular side, potentially explaining its modulating activity. Structural analysis reveals a set of shared themes and variations for inhibitor and modulator recognition that will guide development of the next-generation substrate-selective inhibitors.
中文翻译:

小分子药物抑制和调节γ-分泌酶的结构基础
γ-分泌酶抑制剂 (GSI) 和调节剂 (GSM) 的开发代表了阿尔茨海默病 (AD) 和癌症的有吸引力的治疗机会。然而,这些 GSI 和 GSM 如何靶向 γ-分泌酶在很大程度上仍然未知。在这里,我们报告了人类 γ-分泌酶的冷冻电子显微镜 (cryo-EM) 结构,它们分别与两个 GSI 临床候选药物 Semagacestat 和 Avagacestat、过渡态模拟 GSI L685,458 和经典 GSM E2012 结合,总分辨率为 2.6 –3.1 埃。值得注意的是,每个 GSI 在早老素 1 (PS1) 上占据相同的一般位置,该位置容纳来自淀粉样前体蛋白或 Notch 的 β 链,干扰底物募集。L685,458 直接协调 PS1 的两个催化天冬氨酸残基。E2012 与细胞外 γ-分泌酶的变构位点结合,可能解释其调节活性。结构分析揭示了抑制剂和调节剂识别的一组共享主题和变化,这将指导下一代底物选择性抑制剂的开发。


























 京公网安备 11010802027423号
京公网安备 11010802027423号