Water Research ( IF 12.8 ) Pub Date : 2020-12-27 , DOI: 10.1016/j.watres.2020.116791 Yi Cai , Jennifer N. Apell , Nicholas C. Pflug , Kristopher McNeill , Ulla E. Bollmann
|
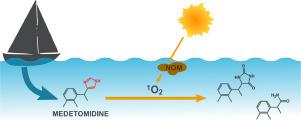
Medetomidine has been authorized in ship hull paints as an antifouling biocide under the biocidal product regulation in Europe since 2016. Its release into marine systems causes concerns over persistence and toxicity. However, the environmental fate of medetomidine has not been fully investigated. In this study, the photodegradation of medetomidine under natural sunlight conditions was investigated using collected coastal and sea waters. In addition, the phototransformation of medetomidine with reactive species (i.e., singlet oxygen, excited triplet state organic matter, and hydroxyl radicals) under UVA light was examined. Photoproducts were isolated by high-performance liquid chromatography (HPLC), identified by a combination of nuclear magnetic resonance (NMR) spectroscopy and time-of-flight mass spectrometry (qTOF), and reaction mechanisms were proposed. The results show that medetomidine is a neutral base (pKa of protonated form = 7.2) that leads to two different protonation states in the aquatic environment. Photodegradation of neutral medetomidine was dominated by reaction with singlet oxygen, while protonated medetomidine was relatively photostable. The contribution of reactive species to the overall photodegradation of neutral medetomidine was calculated to provide an assessment of phototransformation of medetomidine. The half-live of medetomidine was < 1.5 days in natural waters (pHcoastal = 8.3; pHsea = 8.1) under sunlit near-surface conditions, suggesting that it is not persistent in the aquatic environment. Because medetomidine has a relatively short half-life in sunlit aquatic ecosystems, a number of products, such as 2-(2,3-dimethylphenyl)propanamide, can be formed by photochemical reactions of medetomidine, with unknown consequences for marine and coastal waters.
中文翻译:

美托咪定在沿海和海洋环境中的光化学命运
自2016年以来,根据欧洲杀生物产品法规,美托咪定已被授权用于船体涂料中作为防污杀生物剂。其释放到海洋系统中引起持久性和毒性问题。然而,美托咪定的环境命运尚未得到充分研究。在这项研究中,使用收集的沿海和海水调查了美托咪定在自然阳光条件下的光降解。另外,检查了美托咪定在UVA光下与反应性物种(即,单线态氧,激发的三线态有机物和羟基自由基)的光转化。通过高效液相色谱(HPLC)分离光产物,并通过核磁共振(NMR)光谱和飞行时间质谱(qTOF)进行鉴定,并提出了反应机理。结果显示美托咪定为中性碱(p质子化形式的K a = 7.2)在水生环境中导致两种不同的质子化状态。中性美托咪定的光降解作用主要是通过与单线态氧的反应,而质子化的美托咪定则相对光稳定。计算反应性物质对中性美托咪定的总体光降解的贡献,以提供对美托咪定的光转化的评估。美托咪定在天然水域中的半衰期少于1.5天(pH沿海 = 8.3; pH海上 = 8.1)在阳光照射下的近地表条件下,表明它在水生环境中不是持久存在的。由于美托咪定在日光下的水生生态系统中具有相对较短的半衰期,因此美托咪定的光化学反应可以形成许多产品,例如2-(2,3-二甲基苯基)丙酰胺,对海洋和沿海水域的影响未知。


























 京公网安备 11010802027423号
京公网安备 11010802027423号