Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of (S)‐3‐methyleneglutamic acid and its N‐Fmoc derivative via Michael addition–elimination reaction of chiral glycine Ni (II) complex with enol tosylates
Chirality ( IF 2 ) Pub Date : 2020-12-26 , DOI: 10.1002/chir.23291 Yuhei Shigeno 1 , Jianlin Han 2 , Vadim A. Soloshonok 3, 4 , Hiroki Moriwaki 5 , Wataru Fujiwara 1 , Hiroyuki Konno 1
Chirality ( IF 2 ) Pub Date : 2020-12-26 , DOI: 10.1002/chir.23291 Yuhei Shigeno 1 , Jianlin Han 2 , Vadim A. Soloshonok 3, 4 , Hiroki Moriwaki 5 , Wataru Fujiwara 1 , Hiroyuki Konno 1
Affiliation
|
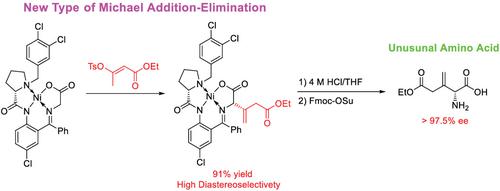
The use of chiral Ni (II)‐complexes of glycine Schiff bases has recently emerged as a leading methodology for asymmetric synthesis of structurally diverse Tailor‐Made Amino Acids™, playing a key role in the design of modern pharmaceuticals. Here, we report first example of enantioselective preparation of (S)‐3‐methyleneglutamic acid and its N‐Fmoc derivative via a new type of Michael addition–elimination reaction between chiral nucleophilic glycine equivalent and enol tosylates. This reaction was found to proceed with excellent yield (91%) and diastereoselectivity (>99/1 de) allowing straightforward asymmetric synthesis of (S)‐3‐methyleneglutamic acid derivatives and analogues. The observed results bode well for general application of this Ni (II) complex approach for preparation and biological studies of this previously unknown type of Tailor‐Made Amino Acids™
中文翻译:

手性甘氨酸镍(II)配合物与烯醇甲苯磺酸酯的迈克尔加成-消除反应不对称合成(S)-3-亚甲基谷氨酸及其N-Fmoc衍生物
最近,使用甘氨酸席夫碱的手性Ni(II)配合物已成为一种不对称合成结构多样的Tailor-Made氨基酸™的领先方法,在现代药物设计中发挥了关键作用。在这里,我们报告通过手性亲核甘氨酸当量与烯醇甲苯磺酸盐之间的新型迈克尔加成-消除反应,对映体选择性制备(S)-3-亚甲基谷氨酸及其N- Fmoc衍生物的第一个例子。已发现该反应以优异的收率(91%)和非对映选择性(> 99/1 de)进行,可以直接合成(S)-3-亚甲基谷氨酸衍生物和类似物。观察到的结果预示着这种镍(II)复杂方法在制备和生物学研究这种未知类型的Tailor-Made氨基酸™中的普遍应用前景良好。
更新日期:2021-02-10
中文翻译:

手性甘氨酸镍(II)配合物与烯醇甲苯磺酸酯的迈克尔加成-消除反应不对称合成(S)-3-亚甲基谷氨酸及其N-Fmoc衍生物
最近,使用甘氨酸席夫碱的手性Ni(II)配合物已成为一种不对称合成结构多样的Tailor-Made氨基酸™的领先方法,在现代药物设计中发挥了关键作用。在这里,我们报告通过手性亲核甘氨酸当量与烯醇甲苯磺酸盐之间的新型迈克尔加成-消除反应,对映体选择性制备(S)-3-亚甲基谷氨酸及其N- Fmoc衍生物的第一个例子。已发现该反应以优异的收率(91%)和非对映选择性(> 99/1 de)进行,可以直接合成(S)-3-亚甲基谷氨酸衍生物和类似物。观察到的结果预示着这种镍(II)复杂方法在制备和生物学研究这种未知类型的Tailor-Made氨基酸™中的普遍应用前景良好。

























 京公网安备 11010802027423号
京公网安备 11010802027423号