Journal of Proteomics ( IF 3.3 ) Pub Date : 2020-12-26 , DOI: 10.1016/j.jprot.2020.104083 Alberto Padilla 1 , Sanaz Dovell 2 , Olga Chesnokov 3 , Mickelene Hoggard 4 , Andrew V Oleinikov 3 , Frank Marí 4
|
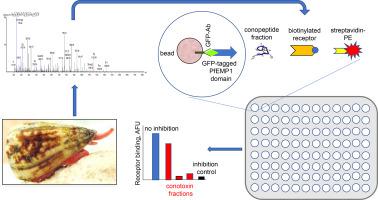
Using high-throughput BioPlex assays, we determined that six fractions from the venom of Conus nux inhibit the adhesion of various recombinant PfEMP-1 protein domains (PF08_0106 CIDR1α3.1, PF11_0521 DBL2β3, and PFL0030c DBL3X and DBL5e) to their corresponding receptors (CD36, ICAM-1, and CSA, respectively). The protein domain-receptor interactions permit P. falciparum-infected erythrocytes (IE) to evade elimination in the spleen by adhering to the microvasculature in various organs including the placenta. The sequences for the main components of the fractions, determined by tandem mass spectrometry, yielded four T-superfamily conotoxins, one (CC-Loop-CC) with I-IV, II-III connectivity and three (CC-Loop-CXaaC) with a I-III, II-IV connectivity. The 3D structure for one of the latter, NuxVA = GCCPAPLTCHCVIY, revealed a novel scaffold defined by double turns forming a hairpin-like structure stabilized by the two disulfide bonds. Two other main fraction components were a miniM conotoxin, and a O2-superfamily conotoxin with cysteine framework VI/VII. This study is the first one of its kind suggesting the use of conotoxins for developing pharmacological tools for anti-adhesion adjunct therapy against malaria. Similarly, mitigation of emerging diseases like AIDS and COVID-19, can also benefit from conotoxins as inhibitors of protein-protein interactions as treatment.
Biological significance
Among the 850+ species of cone snail species there are hundreds of thousands of diverse venom exopeptides that have been selected throughout several million years of evolution to capture prey and deter predators. They do so by targeting several surface proteins present in target excitable cells. This immense biomolecular library of conopeptides can be explored for potential use as therapeutic leads against persistent and emerging diseases affecting non-excitable systems.
We aim to expand the pharmacological reach of conotoxins/conopeptides by revealing their in vitro capacity to disrupt protein-protein and protein-polysaccharide interactions that directly contribute to pathology of Plasmodium falciparum malaria. This is significant for severe forms of malaria, which might be deadly even after treated with current parasite-killing drugs because of persistent cytoadhesion of P. falciparum infected erythrocytes even when parasites within red blood cells are dead. Anti-adhesion adjunct drugs would de-sequester or prevent additional sequestration of infected erythrocytes and may significantly improve survival of malaria patients. These results provide a lead for further investigations into conotoxins and other venom peptides as potential candidates for anti-adhesion or blockade-therapies.
This study is the first of its kind and it suggests that conotoxins can be developed as pharmacological tools for anti-adhesion adjunct therapy against malaria. Similarly, mitigation of emerging diseases like AIDS and COVID-19, can also benefit from conotoxins as potential inhibitors of protein-protein interactions as treatment.
中文翻译:

圆锥毒液组分抑制恶性疟原虫红细胞膜蛋白1结构域与宿主血管受体的粘附
使用高通量 BioPlex 分析,我们确定了来自马铃薯毒液的六个部分抑制各种重组 PfEMP-1 蛋白结构域( PF08_0106 CIDR1α3.1、PF11_0521 DBL2β3 和 PFL0030c DBL3X 和 DBL5e)与其相应受体(CD36 、ICAM-1 和 CSA)。蛋白质结构域-受体相互作用允许受恶性疟原虫感染的红细胞 (IE) 通过粘附到包括胎盘在内的各种器官中的微脉管系统来逃避脾脏中的消除。级分主要成分的序列,通过串联质谱测定,产生了四种 T 超家族芋螺毒素,一种(CC-Loop-CC)具有 I-IV、II-III 连接性和三种(CC-Loop-CX aaC) 具有 I-III、II-IV 连接性。后者之一的 3D 结构 NuxVA = G CC PAPLT C H C VIY 揭示了一种由双转定义的新型支架,形成由两个二硫键稳定的发夹状结构。其他两种主要组分是 miniM 芋螺毒素和具有半胱氨酸骨架 VI/VII 的 O2 超家族芋螺毒素。这项研究是同类研究中第一个建议使用芋螺毒素开发用于抗疟疾的抗粘连辅助治疗的药理学工具。同样,减轻艾滋病和 COVID-19 等新兴疾病也可以受益于芋螺毒素作为蛋白质-蛋白质相互作用抑制剂的治疗。
生物学意义
在 850 多种锥形蜗牛物种中,有数十万种不同的毒液外肽在数百万年的进化过程中被选择用于捕获猎物并阻止捕食者。他们通过靶向存在于目标可兴奋细胞中的几种表面蛋白来做到这一点。可以探索这个巨大的conopeptides 生物分子库,以潜在地用作针对影响非兴奋系统的持续性和新兴疾病的治疗线索。
我们的目标是通过揭示其体外破坏直接导致恶性疟原虫病理学的蛋白质-蛋白质和蛋白质-多糖相互作用的能力来扩大芋螺毒素/芋螺肽的药理学范围。这对于严重形式的疟疾具有重要意义,由于恶性疟原虫的持续细胞粘附,即使在使用当前的寄生虫杀死药物治疗后也可能是致命的即使红细胞内的寄生虫已经死亡,也会感染红细胞。抗粘连辅助药物将解除隔离或防止感染红细胞的额外隔离,并可能显着提高疟疾患者的生存率。这些结果为进一步研究芋螺毒素和其他毒液肽作为抗粘附或阻断疗法的潜在候选者提供了线索。
这项研究是同类研究中的第一个,它表明芋螺毒素可以开发为抗疟疾的抗粘连辅助治疗的药理学工具。同样,减轻艾滋病和 COVID-19 等新兴疾病也可以受益于芋螺毒素作为蛋白质-蛋白质相互作用的潜在抑制剂作为治疗方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号