International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2020-12-25 , DOI: 10.1016/j.ijms.2020.116512 Naader Alizadeh , Razieh Parchami , Edwin De Pauw , Mahmoud Tabrizchi
|
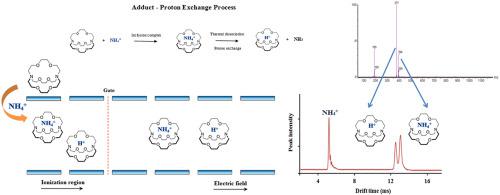
A gas-phase study concerning the interaction between macrocyclic ligands and different molecular ion guests (H3O+, NH4+, CH3NH3+) was performed using ion mobility spectrometry and mass spectrometry. 18-crown-6 (18C6), diaza18-crown-6 (DA18C6) and cryptand [222] (C222) ligands were selected for the investigation of structural properties on the nature of complexes formed. Characterization of complexes was performed by ESI-TWIM-TOFMS. In this study, the importance of the type of donating atom in the ring on complex formation was investigated by comparison of the ion mobility spectra of 18C6 and DA18C6 at various temperatures. Different complexation behaviors of ligands showed that the nature of species formed strongly depends on the structural features of the host. DA18C6 does not demonstrate the adduct formation that is observed for 18C6. This is attributed to the high proton affinity of DA18C6, which serves to preferentially remove a proton from any reactant ion upon introduction to the gas phase. In the case of C222 with a three-dimensional cavity, due to the existence of an inclusive site, an interesting behavior was observed. In C222–H3O+ and C222–CH3NH3+ systems, only proton exchange reaction and an inclusion complex formation (C222.H+) are found, while for C222–NH4+ happens inclusive ammonium complex C222·NH4+ in addition to proton exchange reaction and C222.H+ formation. Encapsulation of ammonium ion in the cage of C222 was attributed to size match between ammonium and host cavity that depends on temperature. The exchange constant, enthalpy and entropy of C222·NH4+ complex formation in the gas phase have been determined.
中文翻译:

使用离子迁移谱和质谱法研究二维和三维大环配体之间加合物和交换络合反应的气相
关于大环配体与不同分子离子客体(H 3 O +,NH 4 +,CH 3 NH 3 +使用离子迁移谱法和质谱法进行)。选择18-crown-6(18C6),diaza18-crown-6(DA18C6)和cryptand [222](C222)配体来研究结构性质对形成的复合物的性质。通过ESI-TWIM-TOFMS进行复合物的表征。在这项研究中,通过比较18C6和DA18C6在不同温度下的离子迁移谱,研究了环中供体原子类型对复合物形成的重要性。配体的不同络合行为表明,所形成物种的性质很大程度上取决于宿主的结构特征。DA18C6没有显示出对18C6观察到的加合物形成。这归因于DA18C6的高质子亲和力,当引入气相时,其用于优先从任何反应离子中除去质子。在具有三维腔体的C222情况下,由于存在包容性位点,因此观察到了有趣的行为。在C222–H中3 Ò +和C222-CH 3 NH 3个+系统中,仅质子交换反应和包合络合物形成(C222.H +)被发现,而C222-NH 4 +恰好包容铵络合物C222·NH 4 +除了质子交换反应与C222.H +形成。铵离子在C222笼中的封装归因于铵和宿主腔之间的尺寸匹配,具体取决于温度。确定了气相中C222·NH 4 +配合物形成的交换常数,焓和熵。

























 京公网安备 11010802027423号
京公网安备 11010802027423号