Archives of Biochemistry and Biophysics ( IF 3.9 ) Pub Date : 2020-12-24 , DOI: 10.1016/j.abb.2020.108731 Nelson A. Araujo , Marta Bruix , Douglas V. Laurents
|
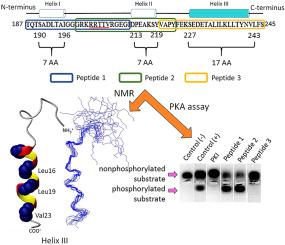
Microbial pathogens, such as Trypanosoma brucei, have an enormous impact on global health and economic systems. Protein kinase A of T. brucei is an attractive drug target as it is an essential enzyme which differs significantly from its human homolog. The hinge region of this protein's regulatory domain is vital for enzymatic function, but its conformation is unknown. Here, the secondary structure of this region has been characterized using NMR and CD spectroscopies. More specifically, three overlapping peptides corresponding to residues T187-I211, G198-Y223 and V220–S245 called peptide 1, peptide 2 and peptide 3, respectively, were studied. The peptide 1 and peptide 2 are chiefly unfolded; only low populations (<10%) of α-helix were detected under the conditions studied. In contrast, the peptide 3 contains a long α-helix whose population is significantly higher; namely, 36% under the conditions studied. Utilizing the dihedral ϕ and ψ angles calculated on the basis of the NMR data, the conformation of the peptide 3 was calculated and revealed an α-helix spanning residues E230-N241. This α-helix showed amphiphilicity and reversible unfolding and refolding upon heating and cooling. Most fascinating, however, is its capacity to inhibit the activity of the catalytic domain of Trypanosoma equiperdum protein kinase A even though it is quite distinct from the canonical inhibitor motif. Based on this property, we advance that peptoids based on the peptide 3 α-helix could be novel leads for developing anti-trypanosomal therapeutics.
中文翻译:

布氏锥虫蛋白激酶A的调节亚基铰链区的紊乱和部分折叠:C接头部分抑制了寄生虫的蛋白激酶A
布鲁氏锥虫等微生物病原体对全球卫生和经济系统具有巨大影响。布氏锥虫的蛋白激酶A是一种有吸引力的药物靶标,因为它是必不可少的酶,与人类同源物有很大不同。该蛋白调节域的铰链区对于酶功能至关重要,但其构象尚不清楚。在这里,该区域的二级结构已使用NMR和CD光谱学进行了表征。更具体地说,研究了三个分别对应于残基T187-I211,G198-Y223和V220-S245的重叠肽,分别称为肽1,肽2和3。肽1和肽2主要是未折叠的。在所研究的条件下,仅检测到少量的α-螺旋(<10%)。相反,肽3含有长的α-螺旋,其种群显着更高。即在研究条件下为36%。利用基于NMR数据计算出的二面角θ和ψ角,计算出肽3的构象,并揭示了跨越α-螺旋残基E230-N241。该α-螺旋显示出两亲性,并且在加热和冷却时可逆地展开和重新折叠。然而,最令人着迷的是其抑制H3O催化结构域活性的能力。锥虫锥虫蛋白激酶A,即使它与典型的抑制剂基序完全不同。基于这一特性,我们提出基于肽3α-螺旋的类肽可能是开发抗锥虫治疗药物的新线索。


























 京公网安备 11010802027423号
京公网安备 11010802027423号