当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibria for Trioctylamine/1-Dodecanol/Citric Acid/Water System at 303.1 and 308.1 K: Experimental Data and Prediction
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-22 , DOI: 10.1021/acs.jced.0c00719 Laura Murcia-Montalvo 1 , Alan D. Pérez 1 , Javier Fontalvo 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-22 , DOI: 10.1021/acs.jced.0c00719 Laura Murcia-Montalvo 1 , Alan D. Pérez 1 , Javier Fontalvo 1
Affiliation
|
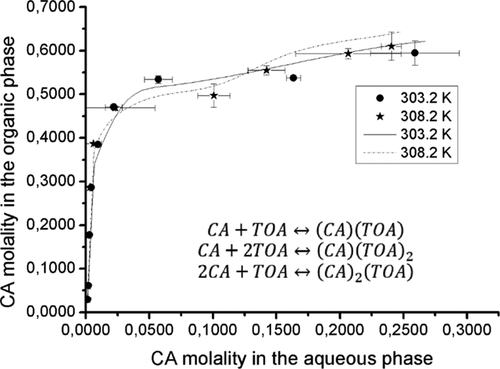
The present work experimentally tests the liquid–liquid equilibria (LLE) of aqueous citric acid (CA) solutions with trioctylamine (TOA) in 1-dodecanol at 303.2 and 308.2 K. A predictive model has been developed based on the experimental data. This model considers the physical dissolution of CA and complexation with TOA. At low CA concentrations, the stoichiometric ratio of acid/amine is (1:1). However, the acid/amine ratios of (1:1), (1:2), and (2:1) are present at high CA concentrations. The equilibrium constants for the different complexes were determined and compared to previous studies, which employed other organic solvents. The equilibrium constants obtained in this study are comparatively higher, making 1-dodecanol an attractive solvation medium. Also, the literature presents LLE experiments with contact times from minutes to hours. Because of this lack of consensus, this work displays experimental evidence to suggest contact and settling times required for experimental evaluation of the equilibria in liquid–liquid systems based on the standard deviation.
中文翻译:

三辛胺/ 1-十二烷醇/柠檬酸/水系统在303.1和308.1 K时的液-液平衡:实验数据和预测
本工作在303.2和308.2 K的条件下,用三辛胺(TOA)在1-十二烷醇中的柠檬酸(CA)水溶液的液-液平衡(LLE)进行了实验测试。已基于实验数据建立了预测模型。该模型考虑了CA的物理溶解和与TOA的络合。在低CA浓度下,酸/胺的化学计量比为(1:1)。但是,高CA浓度下的酸/胺比为(1:1),(1:2)和(2:1)。确定了不同络合物的平衡常数,并将其与使用其他有机溶剂的先前研究进行了比较。在这项研究中获得的平衡常数相对较高,使1-十二烷醇成为有吸引力的溶剂化介质。此外,文献还介绍了LLE实验,接触时间从几分钟到几小时不等。
更新日期:2021-01-14
中文翻译:

三辛胺/ 1-十二烷醇/柠檬酸/水系统在303.1和308.1 K时的液-液平衡:实验数据和预测
本工作在303.2和308.2 K的条件下,用三辛胺(TOA)在1-十二烷醇中的柠檬酸(CA)水溶液的液-液平衡(LLE)进行了实验测试。已基于实验数据建立了预测模型。该模型考虑了CA的物理溶解和与TOA的络合。在低CA浓度下,酸/胺的化学计量比为(1:1)。但是,高CA浓度下的酸/胺比为(1:1),(1:2)和(2:1)。确定了不同络合物的平衡常数,并将其与使用其他有机溶剂的先前研究进行了比较。在这项研究中获得的平衡常数相对较高,使1-十二烷醇成为有吸引力的溶剂化介质。此外,文献还介绍了LLE实验,接触时间从几分钟到几小时不等。



























 京公网安备 11010802027423号
京公网安备 11010802027423号