当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Analysis of Multiple Redox Sites in Complexes [M(C5Me5)(Q)(NO)]n, M=Ru or Os, Q=o‐Quinones
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-12-23 , DOI: 10.1002/zaac.202000420 Thomas M. Scherer 1 , Ingo Hartenbach 1 , Falk Lissner 1 , Brigitte Schwederski 1 , Ralph Hübner 1 , Jan Fiedler 2 , Stanislav Záliš 2 , Biprajit Sarkar 1 , Wolfgang Kaim 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-12-23 , DOI: 10.1002/zaac.202000420 Thomas M. Scherer 1 , Ingo Hartenbach 1 , Falk Lissner 1 , Brigitte Schwederski 1 , Ralph Hübner 1 , Jan Fiedler 2 , Stanislav Záliš 2 , Biprajit Sarkar 1 , Wolfgang Kaim 1
Affiliation
|
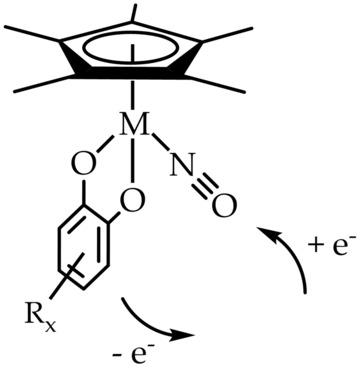
Neutral complexes [MII(C5Me5)(Q2−)(NO+)] with M=Ru or Os and the catecholates Q2− with 3,5‐di‐tert‐butyl (dtbc) or 3,4,5,6‐tetrachloro substituents (tcc) were identified with the oxidation and charge states indicated. Reversible electron transfer was analyzed spectroelectrochemically (EPR, UV‐vis‐NIR, IR), supported by DFT calculations. Reversible reduction was observed for [Ru(C5Me5)(tcc)(NO)] to occur on the nitrosyl ligand to yield the NO radical complex [RuII(C5Me5)(tcc2−)(NO.)]−, whereas the stepwise oxidation yielded o‐semiquinone cations, [MII(C5Me5)(Q.−)(NO+)]+, and dications. The metals remained in the stable low‐spin d6 states, RuII and OsII, respectively. Redox potentials were found to depend strongly on the donor or acceptor substitution pattern at Qn.
中文翻译:

络合物[M(C5Me5)(Q)(NO)] n,M = Ru或Os,Q = o-喹诺酮类中多个氧化还原位点的分析
具有M = Ru或Os的中性络合物[M II(C 5 Me 5)(Q 2−)(NO +)]和具有3,5-二叔丁基(dtbc)或3,4的儿茶酚Q 2−确定了5,5,6-四氯取代基(tcc),并标明了其氧化和电荷状态。在DFT计算的支持下,对可逆电子转移进行了光谱电化学分析(EPR,UV-vis-NIR,IR)。在亚硝酰基配体上观察到[Ru(C 5 Me 5)(tcc)(NO)]可逆还原,生成NO自由基络合物[Ru II(C 5 Me 5)(tcc 2−)(NO)。)] -,而逐步氧化会生成邻半醌阳离子[M II(C 5 Me 5)(Q .-)(NO +)] +和指示剂。金属分别保持在稳定的低旋转d 6状态,即Ru II和Os II。发现氧化还原电势在很大程度上取决于Q n上的供体或受体取代模式。
更新日期:2020-12-23
中文翻译:

络合物[M(C5Me5)(Q)(NO)] n,M = Ru或Os,Q = o-喹诺酮类中多个氧化还原位点的分析
具有M = Ru或Os的中性络合物[M II(C 5 Me 5)(Q 2−)(NO +)]和具有3,5-二叔丁基(dtbc)或3,4的儿茶酚Q 2−确定了5,5,6-四氯取代基(tcc),并标明了其氧化和电荷状态。在DFT计算的支持下,对可逆电子转移进行了光谱电化学分析(EPR,UV-vis-NIR,IR)。在亚硝酰基配体上观察到[Ru(C 5 Me 5)(tcc)(NO)]可逆还原,生成NO自由基络合物[Ru II(C 5 Me 5)(tcc 2−)(NO)。)] -,而逐步氧化会生成邻半醌阳离子[M II(C 5 Me 5)(Q .-)(NO +)] +和指示剂。金属分别保持在稳定的低旋转d 6状态,即Ru II和Os II。发现氧化还原电势在很大程度上取决于Q n上的供体或受体取代模式。


























 京公网安备 11010802027423号
京公网安备 11010802027423号