当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Wor1‐regulated ferroxidases contribute to pigment formation in opaque cells of Candida albicans
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-12-22 , DOI: 10.1002/2211-5463.13070 Baodi Dai 1 , Yinxing Xu 1 , Ning Gao 1 , Jiangye Chen 1
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-12-22 , DOI: 10.1002/2211-5463.13070 Baodi Dai 1 , Yinxing Xu 1 , Ning Gao 1 , Jiangye Chen 1
Affiliation
|
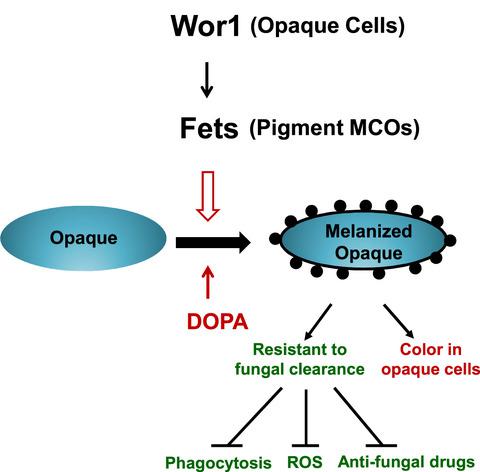
Candida albicans is a harmless commensal resident in the human gut and a prevalent opportunistic pathogen. A key part of its commensalism and pathogenesis is its ability to switch between different morphological forms, including white‐to‐opaque switching. The Wor1 protein was previously identified as a master regulator of white‐to‐opaque switching in mating type locus (MTL) homozygous cells. The mechanisms by which the dark color of the opaque colonies is controlled and the pimpled surface of opaque cells is formed remain unknown. Candida albicans produces melanin pigment in vitro and during infection. However, the molecular mechanism underlying the regulation of melanin production is unclear. In this study, we demonstrated that ferroxidases (Fets) function as pigment multicopper oxidases and regulate the production of dark‐pigmented melanin in opaque cells. The FET genes presented distinct regulation patterns in response to different extracellular stimuli. In YPD (1% yeast extract, 2% peptone and 2% dextrose)‐rich medium, four of the five FET genes were up‐regulated by Wor1, especially at the human body temperature of 37 °C. In minimal medium with low ammonium concentrations, all five FET genes were up‐regulated by Wor1. However, at high ammonium concentrations, some FET genes were down‐regulated by Wor1. Wor1‐up‐regulated Fets contributed to dark pigment formation in opaque colonies, but not to the elongated shape of these opaque cells. Increased melanin externalization was associated with the pimpled surface of the opaque cells. Melanized C. albicans cells were more resistant to fungal clearance. Deletion of the five FET genes completely blocked melanin production in opaque cells and resulted in the generation of white elongated ‘opaque’ cells. In addition, the up‐regulated Fets are important for defense against oxidant attacks. The functional diversity of Fets may reflect the multiple strategies of C. albicans to rapidly adapt to diverse host niches.
中文翻译:

Wor1 调节的亚铁氧化物酶有助于白色念珠菌不透明细胞中的色素形成
白色念珠菌是人类肠道中一种无害的共生菌,是一种普遍的机会性病原体。其共生性和发病机制的一个关键部分是它能够在不同形态形式之间切换,包括从白色到不透明的切换。Wor1 蛋白以前被确定为交配型基因座 ( MTL ) 纯合细胞中白色到不透明转换的主要调节因子。控制不透明集落的深色和形成不透明细胞的丘疹表面的机制仍然未知。白色念珠菌在体外产生黑色素和感染期间。然而,黑色素生成调控的分子机制尚不清楚。在这项研究中,我们证明了亚铁氧化物酶 (Fets) 充当色素多铜氧化酶并调节不透明细胞中深色黑色素的产生。的FET的基因响应于不同的细胞外刺激呈现不同调节图案。在富含 YPD(1% 酵母提取物、2% 蛋白胨和 2% 葡萄糖)的培养基中,五个FET基因中有四个被 Wor1 上调,尤其是在人体温度为 37 °C 时。在低铵浓度的基本培养基中,所有五个FET基因都被 Wor1 上调。然而,在高铵浓度下,一些FET基因被 Wor1 下调。Wor1 上调的 Fets 有助于不透明菌落中暗色素的形成,但不会导致这些不透明细胞的拉长形状。增加的黑色素外化与不透明细胞的丘疹表面有关。黑色化的白色念珠菌细胞对真菌清除更具抵抗力。五个FET基因的缺失完全阻止了不透明细胞中黑色素的产生,并导致产生白色细长的“不透明”细胞。此外,上调的 Fets 对于防御氧化剂攻击很重要。Fets 的功能多样性可能反映了白色念珠菌快速适应不同宿主生态位的多种策略。
更新日期:2020-12-22
中文翻译:

Wor1 调节的亚铁氧化物酶有助于白色念珠菌不透明细胞中的色素形成
白色念珠菌是人类肠道中一种无害的共生菌,是一种普遍的机会性病原体。其共生性和发病机制的一个关键部分是它能够在不同形态形式之间切换,包括从白色到不透明的切换。Wor1 蛋白以前被确定为交配型基因座 ( MTL ) 纯合细胞中白色到不透明转换的主要调节因子。控制不透明集落的深色和形成不透明细胞的丘疹表面的机制仍然未知。白色念珠菌在体外产生黑色素和感染期间。然而,黑色素生成调控的分子机制尚不清楚。在这项研究中,我们证明了亚铁氧化物酶 (Fets) 充当色素多铜氧化酶并调节不透明细胞中深色黑色素的产生。的FET的基因响应于不同的细胞外刺激呈现不同调节图案。在富含 YPD(1% 酵母提取物、2% 蛋白胨和 2% 葡萄糖)的培养基中,五个FET基因中有四个被 Wor1 上调,尤其是在人体温度为 37 °C 时。在低铵浓度的基本培养基中,所有五个FET基因都被 Wor1 上调。然而,在高铵浓度下,一些FET基因被 Wor1 下调。Wor1 上调的 Fets 有助于不透明菌落中暗色素的形成,但不会导致这些不透明细胞的拉长形状。增加的黑色素外化与不透明细胞的丘疹表面有关。黑色化的白色念珠菌细胞对真菌清除更具抵抗力。五个FET基因的缺失完全阻止了不透明细胞中黑色素的产生,并导致产生白色细长的“不透明”细胞。此外,上调的 Fets 对于防御氧化剂攻击很重要。Fets 的功能多样性可能反映了白色念珠菌快速适应不同宿主生态位的多种策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号