当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into the rhodium–copper cascade catalyzed dual C–H annulation of indoles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-10 , DOI: 10.1039/d0qo01332c Xiaoqian He 1, 2, 3, 4, 5 , Lei Zhu 1, 2, 3, 4, 5 , Dan Heng 1, 2, 3, 4, 5 , Fenru Liu 1, 2, 3, 4, 5 , Shihan Liu 1, 2, 3, 4, 5 , Kangbao Zhong 1, 2, 3, 4, 5 , Chunhui Shan 5, 6, 7, 8 , Ruopeng Bai 1, 2, 3, 4, 5 , Yu Lan 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-10 , DOI: 10.1039/d0qo01332c Xiaoqian He 1, 2, 3, 4, 5 , Lei Zhu 1, 2, 3, 4, 5 , Dan Heng 1, 2, 3, 4, 5 , Fenru Liu 1, 2, 3, 4, 5 , Shihan Liu 1, 2, 3, 4, 5 , Kangbao Zhong 1, 2, 3, 4, 5 , Chunhui Shan 5, 6, 7, 8 , Ruopeng Bai 1, 2, 3, 4, 5 , Yu Lan 1, 2, 3, 4, 5
Affiliation
|
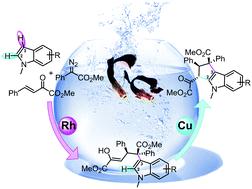
Density functional theory (DFT) calculations have been performed to provide mechanistic insight into the Rh/Cu co-catalyzed multicomponent annulation of indoles, diazo compounds, and α,β-unsaturated esters. Indole can undergo electrophilic attack by a dirhodium–carbene complex to form a cyclopropane intermediate, which is transferred to an enolate by deprotonation. A dimetallic Michael-type addition reaction is proposed by DFT calculation, where the diastereoselectivity is controlled by the interaction energy between the incoming α,β-unsaturated ester and enolate nucleophile. In copper catalysis, an intramolecular oxidation by copper enolate/copper ketonate resonance is revealed, by which copper enolate is partially oxidized to an α-carbonyl radical. Therefore, intramolecular radical addition with the indole moiety achieves annulation with the formation of a C3 radical in dearomatic indole. Oxidative hydrogen atom transfer then gives the aromatic annulation product by using excess copper chloride.
中文翻译:

铑-铜级联催化吲哚双C-H圆环的机理研究
已经进行了密度泛函理论(DFT)计算,以提供对Rh / Cu共催化的吲哚,重氮化合物和α,β-不饱和酯的多组分环合的机理的见解。吲哚可以通过二价carb-卡宾络合物进行亲电攻击,形成环丙烷中间体,然后通过去质子化将其转移至烯醇化物中。通过DFT计算提出了双金属迈克尔型加成反应,其中非对映选择性受传入的α,β-不饱和酯与烯醇式亲核试剂之间的相互作用能控制。在铜催化中,揭示了由烯醇铜/铜酮酸酯共振引起的分子内氧化,由此烯醇铜被部分氧化成α-羰基自由基。因此,带有吲哚部分的分子内自由基加成可实现环化反应,并在脱芳族吲哚中形成C3自由基。然后,通过使用过量的氯化铜,氧化性氢原子转移得到芳族环化产物。
更新日期:2020-12-21
中文翻译:

铑-铜级联催化吲哚双C-H圆环的机理研究
已经进行了密度泛函理论(DFT)计算,以提供对Rh / Cu共催化的吲哚,重氮化合物和α,β-不饱和酯的多组分环合的机理的见解。吲哚可以通过二价carb-卡宾络合物进行亲电攻击,形成环丙烷中间体,然后通过去质子化将其转移至烯醇化物中。通过DFT计算提出了双金属迈克尔型加成反应,其中非对映选择性受传入的α,β-不饱和酯与烯醇式亲核试剂之间的相互作用能控制。在铜催化中,揭示了由烯醇铜/铜酮酸酯共振引起的分子内氧化,由此烯醇铜被部分氧化成α-羰基自由基。因此,带有吲哚部分的分子内自由基加成可实现环化反应,并在脱芳族吲哚中形成C3自由基。然后,通过使用过量的氯化铜,氧化性氢原子转移得到芳族环化产物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号