当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of residual disinfectants on the redox speciation of lead(II)/(IV) minerals in drinking water distribution systems
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2020-12-15 , DOI: 10.1039/d0ew00706d Sumant Avasarala 1 , John Orta 1 , Michael Schaefer 2 , Macon Abernathy 2 , Samantha Ying 2 , Haizhou Liu 1
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2020-12-15 , DOI: 10.1039/d0ew00706d Sumant Avasarala 1 , John Orta 1 , Michael Schaefer 2 , Macon Abernathy 2 , Samantha Ying 2 , Haizhou Liu 1
Affiliation
|
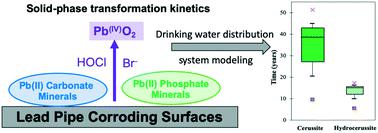
This study investigated the reaction kinetics on the oxidative transformation of lead(II) minerals by free chlorine (HOCl) and free bromine (HOBr) in drinking water distribution systems. According to chemical equilibrium predictions, lead(II) carbonate minerals, cerussite PbCO3(s) and hydrocerussite Pb3(CO3)2(OH)2(s), and lead(II) phosphate mineral, chloropyromorphite Pb5(PO4)3Cl(s) are formed in drinking water distribution systems in the absence and presence of phosphate, respectively. X-ray absorption near edge spectroscopy (XANES) data showed that at pH 7 and a 10 mM alkalinity, the majority of cerussite and hydrocerussite was oxidized to lead(IV) mineral PbO2(s) within 120 minutes of reaction with chlorine (3 : 1 Cl2 : Pb(II) molar ratio). In contrast, very little oxidation of chloropyromorphite occurred. Under similar conditions, oxidation of lead(II) carbonate and phosphate minerals by HOBr exhibited a reaction kinetics that was orders of magnitude faster than by HOCl. Their end oxidation products were identified as mainly plattnerite β-PbO2(s) and trace amounts of scrutinyite α-PbO2(s) based on X-ray diffraction (XRD) and extended X-ray absorption fine structure (EXAFS) spectroscopic analysis. A kinetic model was established based on the solid-phase experimental data. The model predicted that in real drinking water distribution systems, it takes 0.6–1.2 years to completely oxidize Pb(II) minerals in the surface layer of corrosion scales to PbO2(s) by HOCl without phosphate, but only 0.1–0.2 years in the presence of bromide (Br−) due the catalytic effects of HOBr generation. The model also predicts that the addition of phosphate will significantly inhibit Pb(II) mineral oxidation by HOCl, but only be modestly effective in the presence of Br−. This study provides insightful understanding on the effect of residual disinfectant on the oxidation of lead corrosion scales and strategies to prevent lead release from drinking water distribution systems.
中文翻译:

残留消毒剂对饮用水分配系统中铅(II)/(IV)矿物质氧化还原形态的影响
本研究调查了饮用水分配系统中游离氯 (HOCl) 和游离溴 (HOBr)对铅 ( II ) 矿物质氧化转化的反应动力学。根据化学平衡预测,铅( II )碳酸盐矿物,蜡石PbCO 3(s)和水蜡石Pb 3 (CO 3 ) 2 (OH) 2(s),以及磷酸铅( II )矿物,氯焦吗啉Pb 5 (PO 4) ) 3氯(s)分别在不存在和存在磷酸盐的情况下在饮用水分配系统中形成。X 射线吸收近边光谱 (XANES) 数据显示,在 pH 值为 7 和碱度为 10 mM 的情况下,大部分蜡矿和水蜡矿在与氯反应 120 分钟内被氧化为铅 ( IV ) 矿物 PbO 2(s) (3 : 1 Cl 2 : Pb( II ) 摩尔比)。相比之下,几乎没有发生氯焦吗啉的氧化。在类似条件下,HOBr 对碳酸铅 ( II ) 和磷酸盐矿物的氧化表现出比 HOCl 快几个数量级的反应动力学。他们的最终氧化产物被确定为主要是 plattnerite β-PbO 2(s)以及基于 X 射线衍射 (XRD) 和扩展 X 射线吸收精细结构 (EXAFS) 光谱分析的微量细查矿 α-PbO 2(s)。基于固相实验数据建立了动力学模型。该模型预测,在实际饮用水配水系统中,不含磷酸盐的HOCl将腐蚀水垢表层的Pb( II )矿物完全氧化为PbO 2(s)需要0 .6- 1.2年,而在0.1-0.2年的时间内由于 HOBr 生成的催化作用,溴化物 (Br - )的存在。该模型还预测,磷酸盐的添加将显着抑制HOCl 对Pb( II ) 矿物的氧化,但在 Br 存在的情况下仅适度有效-。这项研究提供了对残留消毒剂对铅腐蚀水垢氧化的影响的深刻理解,以及防止饮用水分配系统释放铅的策略。
更新日期:2020-12-21
中文翻译:

残留消毒剂对饮用水分配系统中铅(II)/(IV)矿物质氧化还原形态的影响
本研究调查了饮用水分配系统中游离氯 (HOCl) 和游离溴 (HOBr)对铅 ( II ) 矿物质氧化转化的反应动力学。根据化学平衡预测,铅( II )碳酸盐矿物,蜡石PbCO 3(s)和水蜡石Pb 3 (CO 3 ) 2 (OH) 2(s),以及磷酸铅( II )矿物,氯焦吗啉Pb 5 (PO 4) ) 3氯(s)分别在不存在和存在磷酸盐的情况下在饮用水分配系统中形成。X 射线吸收近边光谱 (XANES) 数据显示,在 pH 值为 7 和碱度为 10 mM 的情况下,大部分蜡矿和水蜡矿在与氯反应 120 分钟内被氧化为铅 ( IV ) 矿物 PbO 2(s) (3 : 1 Cl 2 : Pb( II ) 摩尔比)。相比之下,几乎没有发生氯焦吗啉的氧化。在类似条件下,HOBr 对碳酸铅 ( II ) 和磷酸盐矿物的氧化表现出比 HOCl 快几个数量级的反应动力学。他们的最终氧化产物被确定为主要是 plattnerite β-PbO 2(s)以及基于 X 射线衍射 (XRD) 和扩展 X 射线吸收精细结构 (EXAFS) 光谱分析的微量细查矿 α-PbO 2(s)。基于固相实验数据建立了动力学模型。该模型预测,在实际饮用水配水系统中,不含磷酸盐的HOCl将腐蚀水垢表层的Pb( II )矿物完全氧化为PbO 2(s)需要0 .6- 1.2年,而在0.1-0.2年的时间内由于 HOBr 生成的催化作用,溴化物 (Br - )的存在。该模型还预测,磷酸盐的添加将显着抑制HOCl 对Pb( II ) 矿物的氧化,但在 Br 存在的情况下仅适度有效-。这项研究提供了对残留消毒剂对铅腐蚀水垢氧化的影响的深刻理解,以及防止饮用水分配系统释放铅的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号