当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NMR Structure Elucidation of Naphthoquinones from Quambalaria cyanescens
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-12-19 , DOI: 10.1021/acs.jnatprod.0c00930 Eliška Procházková 1 , Oleksandr Kucherak 2 , Eva Stodůlková 3 , Zdeněk Tošner 2 , Ivana Císařová 2 , Miroslav Flieger 3 , Miroslav Kolařík 3 , Ondřej Baszczyňski 2
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-12-19 , DOI: 10.1021/acs.jnatprod.0c00930 Eliška Procházková 1 , Oleksandr Kucherak 2 , Eva Stodůlková 3 , Zdeněk Tošner 2 , Ivana Císařová 2 , Miroslav Flieger 3 , Miroslav Kolařík 3 , Ondřej Baszczyňski 2
Affiliation
|
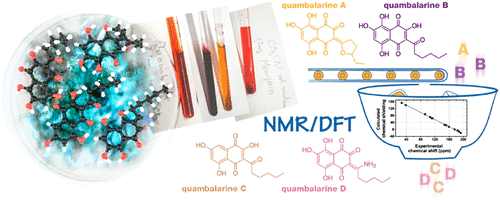
Naphthoquinones isolated from Quambalaria cyanescens (quambalarines) are natural pigments possessing significant cytotoxic and antimicrobial properties. Determining the structure of naphthoquinone compounds is important for the understanding of their biological activities and the informed synthesis of related analogues. Identifying quambalarines is challenging, because they contain a hydroxylated naphthoquinone scaffold and have limited solubility. Here, we report a detailed structural study of quambalarine derivatives, which form strong intramolecular hydrogen bonds (IMHBs) that enable the formation of several tautomers; these tautomers may complicate structural investigation due to their fast interconversion. To investigate tautomeric equilibria and identify new quambalarines, we complemented the experimental NMR spectroscopy data with density functional theory (DFT) calculations.
中文翻译:

紫花青霉中萘醌类化合物的核磁共振结构解析
从Quambalaria cyanescens 中分离出的萘醌(quambararines)是具有显着细胞毒性和抗菌特性的天然色素。确定萘醌化合物的结构对于了解其生物活性和相关类似物的知情合成非常重要。鉴定 quambararines 具有挑战性,因为它们含有羟基化萘醌支架并且溶解度有限。在这里,我们报告了对 quambararine 衍生物的详细结构研究,这些衍生物形成强大的分子内氢键 (IMHBs),能够形成几种互变异构体;由于它们的快速互变,这些互变异构体可能使结构研究复杂化。为了研究互变异构平衡并确定新的 quambararines,我们用密度泛函理论 (DFT) 计算补充了实验 NMR 光谱数据。
更新日期:2021-01-22
中文翻译:

紫花青霉中萘醌类化合物的核磁共振结构解析
从Quambalaria cyanescens 中分离出的萘醌(quambararines)是具有显着细胞毒性和抗菌特性的天然色素。确定萘醌化合物的结构对于了解其生物活性和相关类似物的知情合成非常重要。鉴定 quambararines 具有挑战性,因为它们含有羟基化萘醌支架并且溶解度有限。在这里,我们报告了对 quambararine 衍生物的详细结构研究,这些衍生物形成强大的分子内氢键 (IMHBs),能够形成几种互变异构体;由于它们的快速互变,这些互变异构体可能使结构研究复杂化。为了研究互变异构平衡并确定新的 quambararines,我们用密度泛函理论 (DFT) 计算补充了实验 NMR 光谱数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号