当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Associations between Drug-Induced Adverse Events in Animal Models and Humans Reveal Novel Candidate Safety Targets
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-12-18 , DOI: 10.1021/acs.chemrestox.0c00311 Kathryn A Giblin 1, 2 , Danilo Basili 1 , Avid M Afzal 3 , Lyn Rosenbrier-Ribeiro 4 , Nigel Greene 5 , Ian Barrett 3 , Samantha J Hughes 2 , Andreas Bender 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-12-18 , DOI: 10.1021/acs.chemrestox.0c00311 Kathryn A Giblin 1, 2 , Danilo Basili 1 , Avid M Afzal 3 , Lyn Rosenbrier-Ribeiro 4 , Nigel Greene 5 , Ian Barrett 3 , Samantha J Hughes 2 , Andreas Bender 1
Affiliation
|
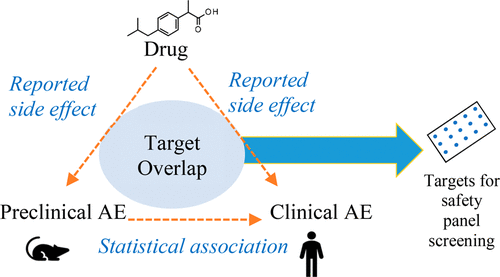
To improve our ability to extrapolate preclinical toxicity to humans, there is a need to understand and quantify the concordance of adverse events (AEs) between animal models and clinical studies. In the present work, we discovered 3011 statistically significant associations between preclinical and clinical AEs caused by drugs reported in the PharmaPendium database of which 2952 were new associations between toxicities encoded by different Medical Dictionary for Regulatory Activities terms across species. To find plausible and testable candidate off-target drug activities for the derived associations, we investigated the genetic overlap between the genes linked to both a preclinical and a clinical AE and the protein targets found to interact with one or more drugs causing both AEs. We discuss three associations from the analysis in more detail for which novel candidate off-target drug activities could be identified, namely, the association of preclinical mutagenicity readouts with clinical teratospermia and ovarian failure, the association of preclinical reflexes abnormal with clinical poor-quality sleep, and the association of preclinical psychomotor hyperactivity with clinical drug withdrawal syndrome. Our analysis successfully identified a total of 77% of known safety targets currently tested in in vitro screening panels plus an additional 431 genes which were proposed for investigation as future safety targets for different clinical toxicities. This work provides new translational toxicity relationships beyond AE term-matching, the results of which can be used for risk profiling of future new chemical entities for clinical studies and for the development of future in vitro safety panels.
中文翻译:

动物模型中药物引起的不良事件与人类之间的新关联揭示了新的候选安全目标
为了提高我们将临床前毒性外推到人类的能力,需要了解和量化动物模型和临床研究之间不良事件 (AE) 的一致性。在目前的工作中,我们发现了 PharmaPendium 数据库中报告的药物引起的临床前和临床 AE 之间的 3011 个统计学上显着的关联,其中 2952 个是由不同物种的监管活动医学词典编码的毒性之间的新关联。为了为衍生关联找到合理且可测试的候选脱靶药物活性,我们研究了与临床前和临床 AE 相关的基因与发现与一种或多种导致两种 AE 的药物相互作用的蛋白质靶标之间的遗传重叠。畸形精子症和卵巢功能衰竭,临床前反射异常与临床睡眠质量差的关联,以及临床前精神运动过度活跃与临床药物戒断综合征的关联。我们的分析成功确定了目前在体外测试的总共 77% 的已知安全目标筛选面板加上额外的 431 个基因,这些基因被提议作为不同临床毒性的未来安全目标进行研究。这项工作提供了超越 AE 术语匹配的新的转化毒性关系,其结果可用于未来临床研究的新化学实体的风险分析和未来体外安全小组的开发。
更新日期:2021-02-15
中文翻译:

动物模型中药物引起的不良事件与人类之间的新关联揭示了新的候选安全目标
为了提高我们将临床前毒性外推到人类的能力,需要了解和量化动物模型和临床研究之间不良事件 (AE) 的一致性。在目前的工作中,我们发现了 PharmaPendium 数据库中报告的药物引起的临床前和临床 AE 之间的 3011 个统计学上显着的关联,其中 2952 个是由不同物种的监管活动医学词典编码的毒性之间的新关联。为了为衍生关联找到合理且可测试的候选脱靶药物活性,我们研究了与临床前和临床 AE 相关的基因与发现与一种或多种导致两种 AE 的药物相互作用的蛋白质靶标之间的遗传重叠。畸形精子症和卵巢功能衰竭,临床前反射异常与临床睡眠质量差的关联,以及临床前精神运动过度活跃与临床药物戒断综合征的关联。我们的分析成功确定了目前在体外测试的总共 77% 的已知安全目标筛选面板加上额外的 431 个基因,这些基因被提议作为不同临床毒性的未来安全目标进行研究。这项工作提供了超越 AE 术语匹配的新的转化毒性关系,其结果可用于未来临床研究的新化学实体的风险分析和未来体外安全小组的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号