Current Organic Chemistry ( IF 2.6 ) Pub Date : 2020-10-31 , DOI: 10.2174/1385272824999200817170058 Enakshi Dinda 1 , Samir Kumar Bhunia 2 , Ranjan Jana 3
|
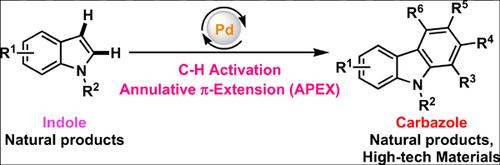
The annulative π-extension (APEX) reactions through C-H bond activation has tremendous potential to access fused aromatic systems from relatively simple aromatic compounds in a single step. This state-of-the-art technique has the ability to streamline the synthesis of functionalized materials useful in material science, biomedical research, agroand pharmaceutical industries. Furthermore, C-H activation strategy does not require prefunctionalization steps, which allows for the late-stage modification of the functional molecule with requisite molecular properties. Owing to their unique photophysical properties, carbazoles are widely used in photovoltaic cells, biomedical imaging, fluorescent polymer, etc. It is also ubiquitously found in many natural products, agrochemicals and privileged medicinal scaffolds. Hence, direct conversion of easily accessible indole to carbazole remains an active research area. In the last decades, significant advancement has been made to access carbazole moiety directly from indole through cascade C-H activation. The underlying mechanism behind this cascade π-extension strategy is the facile electrophilic metalation at the C-3 position of the indole moiety, 1,2- migration and electro cyclization. In this review, we will discuss recent literature reports for the palladium-catalyzed π-extension of indole to carbazole moiety through C-H bond activation.
中文翻译:

钯催化级联反应,通过C–H键活化将吲哚环化延伸至咔唑
通过CH键活化的环状π延伸(APEX)反应具有巨大的潜力,可通过一步从相对简单的芳族化合物中获得稠合的芳族体系。这种最先进的技术具有简化用于材料科学,生物医学研究,农业和制药业的功能化材料的合成的能力。此外,CH活化策略不需要预功能化步骤,从而可以对功能分子进行后期修饰,使其具有必要的分子特性。咔唑因其独特的光物理性质而被广泛用于光伏电池,生物医学成像,荧光聚合物等。在许多天然产品,农用化学品和特有的药物支架中也普遍发现了咔唑。因此,易于获得的吲哚直接转化为咔唑仍然是一个活跃的研究领域。在最近的几十年中,通过级联CH活化直接从吲哚接近咔唑部分已经取得了重大进展。该级联π-延伸策略背后的潜在机理是在吲哚部分的C-3位置上容易的亲电子金属化,1,2-迁移和电环化。在这篇综述中,我们将讨论有关通过CH键激活钯催化的吲哚向咔唑部分的π延伸的文献报道。该级联π-延伸策略背后的潜在机理是在吲哚部分的C-3位置上容易的亲电子金属化,1,2-迁移和电环化。在这篇综述中,我们将讨论有关通过CH键激活钯催化的吲哚向咔唑部分的π延伸的文献报道。该级联π-延伸策略背后的潜在机理是在吲哚部分的C-3位置上容易的亲电子金属化,1,2-迁移和电环化。在这篇综述中,我们将讨论有关通过CH键激活钯催化的吲哚向咔唑部分的π延伸的文献报道。



























 京公网安备 11010802027423号
京公网安备 11010802027423号